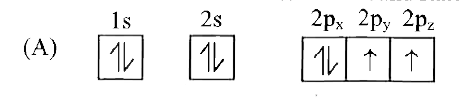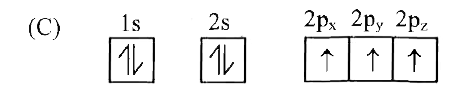A
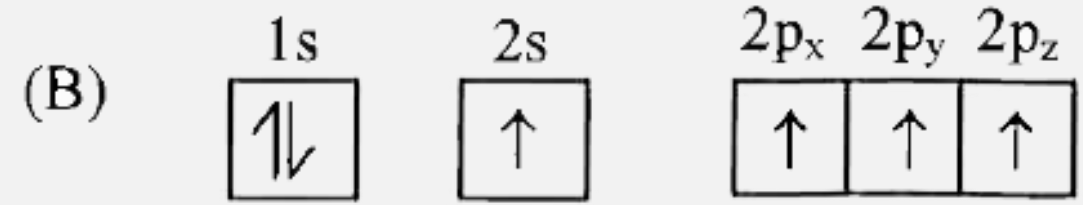
B
C
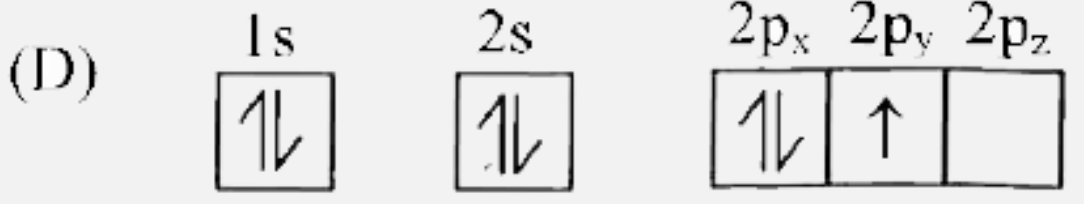
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TARGET PUBLICATION-NATURE OF CHEMICAL BOND-Evaluation test
- Which of the following is the electronic configuration of nitrogen ato...
Text Solution
|
- In a homologous series, the percentage of 's' character .
Text Solution
|
- Which of the following molecule has two p-orbitals unused?
Text Solution
|
- Energy required to dissociate 4g of gaseous hydrogen into free gaseous...
Text Solution
|
- What happens when aluminium reacts with NaOH in presence of water mole...
Text Solution
|
- What is the geometrical shape of [Pt(Cl)(4)]^(2-)?
Text Solution
|
- Stability of the species Li(2), Li(2)^(-) and Li(2)^(+) increases in t...
Text Solution
|
- Which of the following is NOT used to represent bond angles ?
Text Solution
|
- The number of sigma bonds in CH(2)=CH - CH = CH - C -= CH is .
Text Solution
|
- The stability of ionic crystal depends principally on
Text Solution
|
- An example for an electron deficient molecule is .
Text Solution
|
- Octahedral molecular shape exists in hybridisation.
Text Solution
|
- The species having bond angles of 109^(@)28 is .
Text Solution
|
- The bond angle in ammonia is 107^(@)18 due to .
Text Solution
|
- A molecule is stable when the number of bonding orbitals is .
Text Solution
|
- Which of the following molecules CANNOT exist under normal conditions?
Text Solution
|
- The electronegativity values of C,H,O,N and S are 2.5, 2.1, 3.5, 3.0 a...
Text Solution
|
- Find the CORRECT set with respect to molecule, hybridisation and shape...
Text Solution
|
- If in a polar molecule, the ionic charge is 2.6 xx 10^(-10) e.s.u. and...
Text Solution
|
- Which of the following does NOT show hydrogen bonding?
Text Solution
|
- Which of the following theory provides good explanation about the para...
Text Solution
|