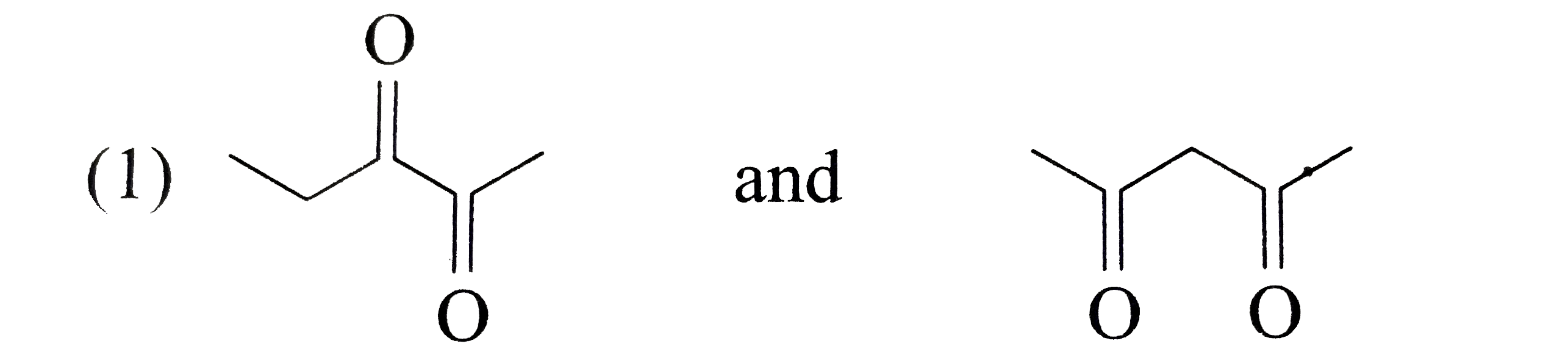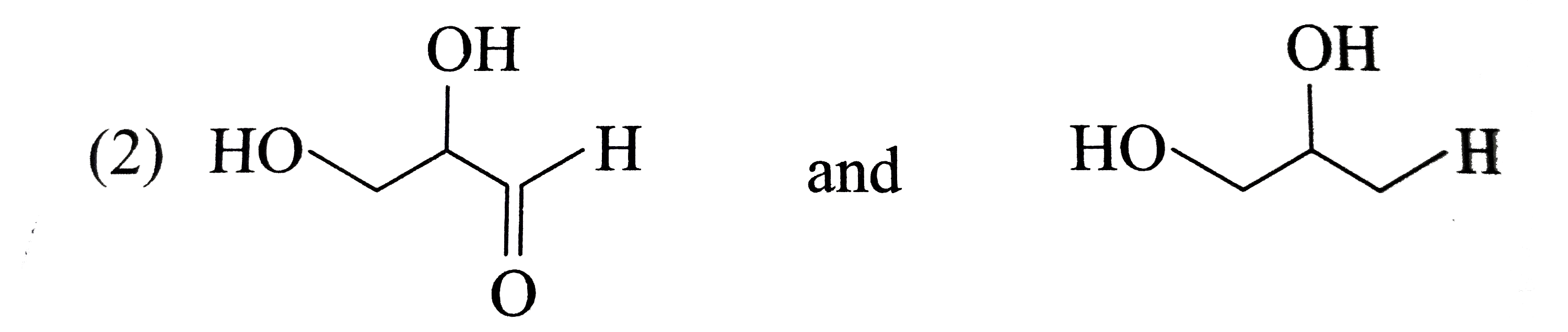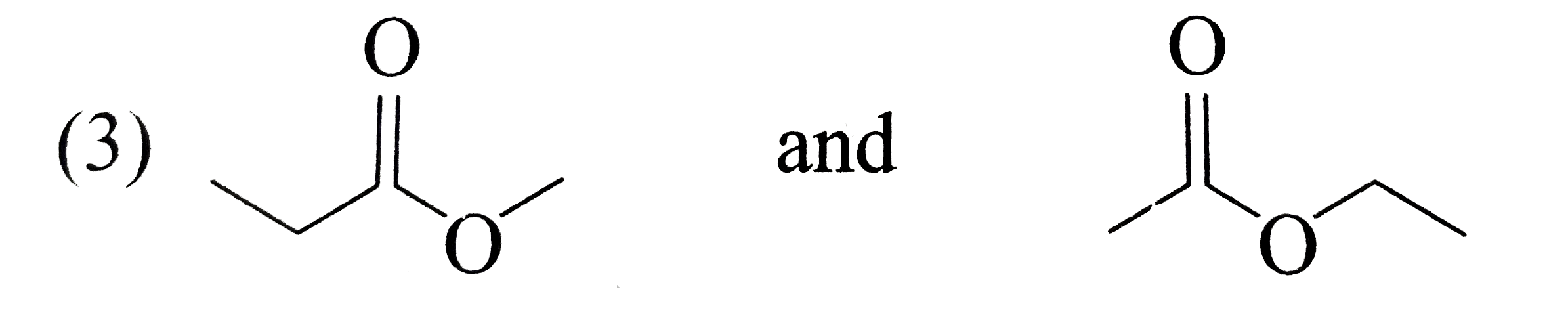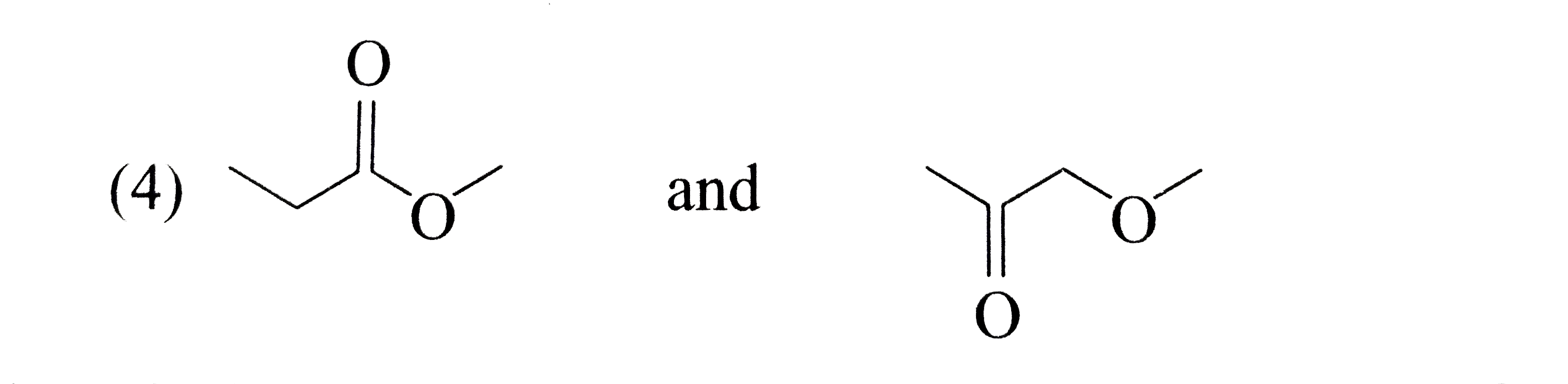A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ISOMERISM-Question Bank
- Strutural isomers C2H5OH and CH3OCH3 have the same value of
Text Solution
|
- The number of ether metamers represented by the formula C4H(10)O is
Text Solution
|
- The number of possible structural isomers of C4H(10)O is
Text Solution
|
- The total number of possible isomeric trimethylbenzenes is
Text Solution
|
- The enolic form of acetone contains:
Text Solution
|
- How many consitutional isomers are possible for the arene C9H(12)?
Text Solution
|
- The number of possible structural isomers of C4H(10)O is
Text Solution
|
- The number of aromatic structures possible for the molecular formula C...
Text Solution
|
- A molecule of urea can show
Text Solution
|
- Total number of ethers possible with the molecular formula C5H(12)O ex...
Text Solution
|
- Which of the following pairs of compounds are functional isomers?
Text Solution
|
- Which of the following does not exhibit tautomerism?
Text Solution
|
- The number of carboxylic esters with the formula C5H(10)O2 exhibiting ...
Text Solution
|
- The total number of dimethylphenols having the molecular formula C8H(1...
Text Solution
|
- Which one of the following formular does not represents an organic com...
Text Solution
|
- Only two isomeric monochloro derivatives are possible for
Text Solution
|
- Keto-enol tautomerism is observed in
Text Solution
|
- Tautomerism is exhibited by (i) (ii) (iii) (iv)
Text Solution
|
- The enol form of acetone after treatment with D(2)O gives:
Text Solution
|
- Which of the following has the most acidic hydrogen ?
Text Solution
|