Cubic closest packing uses a face centered cubic unit cell.Looking at any one face of the cube, we notice that the face atoms touch the corner atoms along the diagonal of the face but that corner atoms do not touch one another along the edges
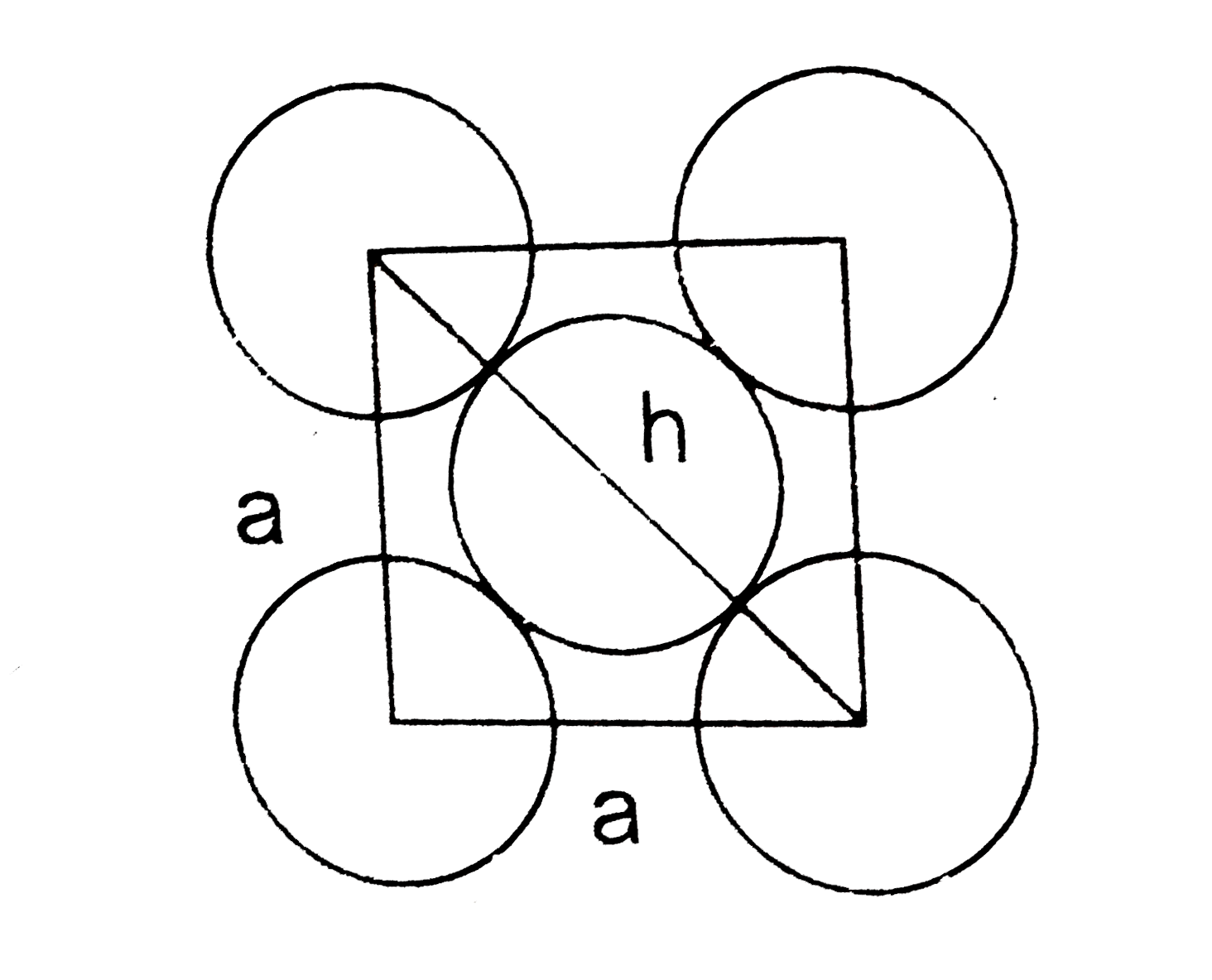
therefore the two atoms closest to each other are those along the face diagonal. One face of the face centered unit cell is shown above. with the atoms touching ,the nearnest neighbour atoms are the ones along the diagonal of the face of the cube , We may visualize the face as consisting of two righ isoscales triangle sharing a common hypotenuse ,`h` and having sides of lengths `a=407` pm. the hypotenuse is equal to twice the centre to centre distance.The hypotaneous can be calculated from the pythagorean theorem `h^(2)=a^(2)+d^(2)` .The length of the hypotaneous equals the square root of the sum of the squares of the sides
`h=sqrt(a^(2)+a^(2))=sqrt(2a^(2))`
`=sqrt(2(407)p m^(2))=576`pm