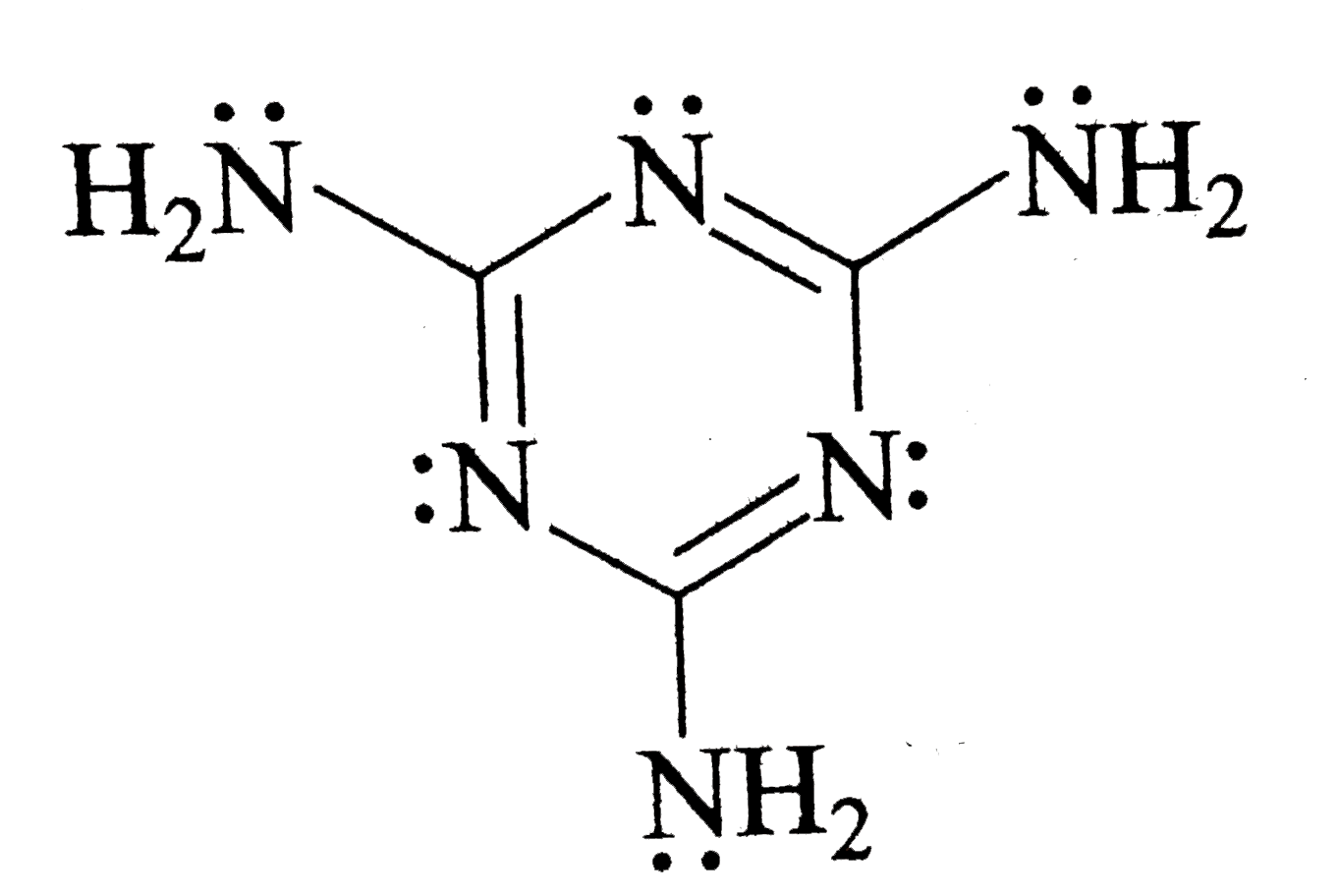A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-POLYMERS-All Questions
- Which one of the following is used to mae 'non - stick' coodware?
Text Solution
|
- Which one is not classified as a condensation polymer? (i) teflon ...
Text Solution
|
- The total number of lone-paris of electrons in melamine is
Text Solution
|
- Caprolactam, a starting material for the manufacture of nylon 6, is pr...
Text Solution
|
- Which one of the following represent nylon 6,6 polymer?
Text Solution
|
- Natural rubber has:
Text Solution
|
- Caprolactam, is used for the manufacture of
Text Solution
|
- Biodegradalbe polymer whichcan be produced from glycine and aminocapro...
Text Solution
|
- Which one of the following is an example of a thermosetting polymer?
Text Solution
|
- Which of the following organic compounds polymerize to form the polyes...
Text Solution
|
- Which is the monomer of neoprene in the following?
Text Solution
|
- Nylon is an example of
Text Solution
|
- Which one of the following is not a condensation polymer?
Text Solution
|
- Which of the following statements is false?
Text Solution
|
- Which one of the following sets forms the biodegrable polymer?
Text Solution
|
- Of the following which one is classified as polyester polymer?
Text Solution
|
- Which of the following structures represents neoprene polymers?
Text Solution
|
- Struchures of some common polymers are given. Which one is not correct...
Text Solution
|
- The straight chain polymer is formed by
Text Solution
|
- Which of the following statement is not ture?
Text Solution
|