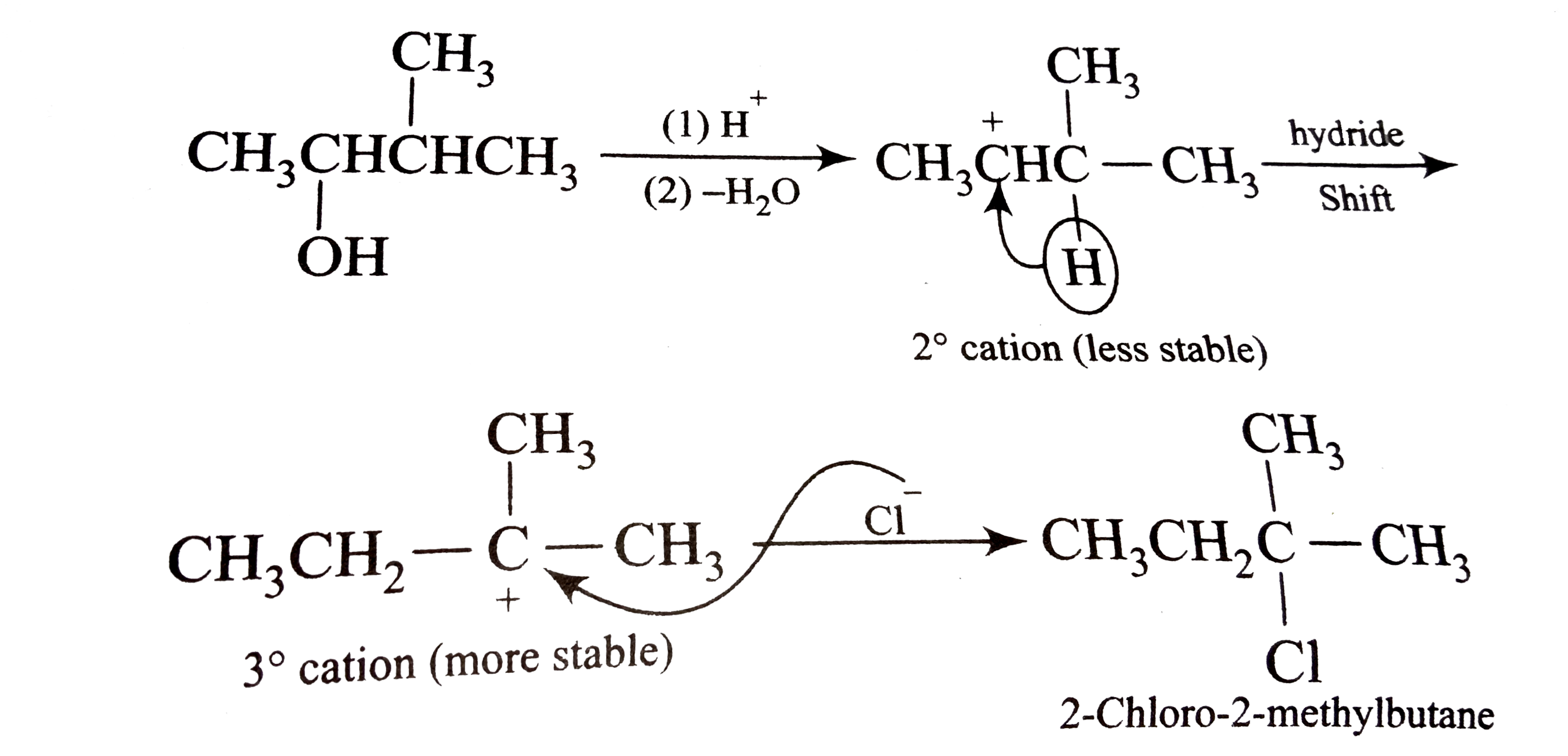
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-Follow -Up Test -6
- (R ) -hexan -2-ol overset("conc.HX ") rarr under S(N)2 conditions t...
Text Solution
|
- The major product formed in the reaction CH(3)-underset(OH)underse...
Text Solution
|
- Trityl chloride (Ph(3)C Cl) is allowed to react with ethyl alcohol in ...
Text Solution
|
- Lucas reagent produces cloudiness immediately with
Text Solution
|
- What will be the order of reactivity of the following alcohols towards...
Text Solution
|
- By which of the following sequence of steps can the alcohol RCH(2)CH(...
Text Solution
|
- Ethyl alcohol is heated with conc.H(2)SO(4).The product formed is
Text Solution
|
- HBr reacts slowest with
Text Solution
|
- 3-Methylbutan-2-ol on reaction with HCl gives predominantly
Text Solution
|
- In the reaction CH(3)-underset(CH(3))underset(|)overset(CH(3))overse...
Text Solution
|
- Which of the following reaction of (S)-CH(3)CHD(OH) proceeds with rete...
Text Solution
|
- Which of th efollowing alcohols are excepted to react with hydrogen ha...
Text Solution
|
- Which of the following statements is not correct ?
Text Solution
|
- Which of the following reagents is preferred to synthesize neopentryl ...
Text Solution
|
- When but -3-en-2-ol reacts with aq HBr , we get
Text Solution
|
- The product of the reaction of Ph(2)CHCh(2)OH with HBr is
Text Solution
|
- Place the following alcohols in decreasing order of rate of dehydratio...
Text Solution
|
- Place the following benzyl alcohols in decreasing order of reaction ra...
Text Solution
|