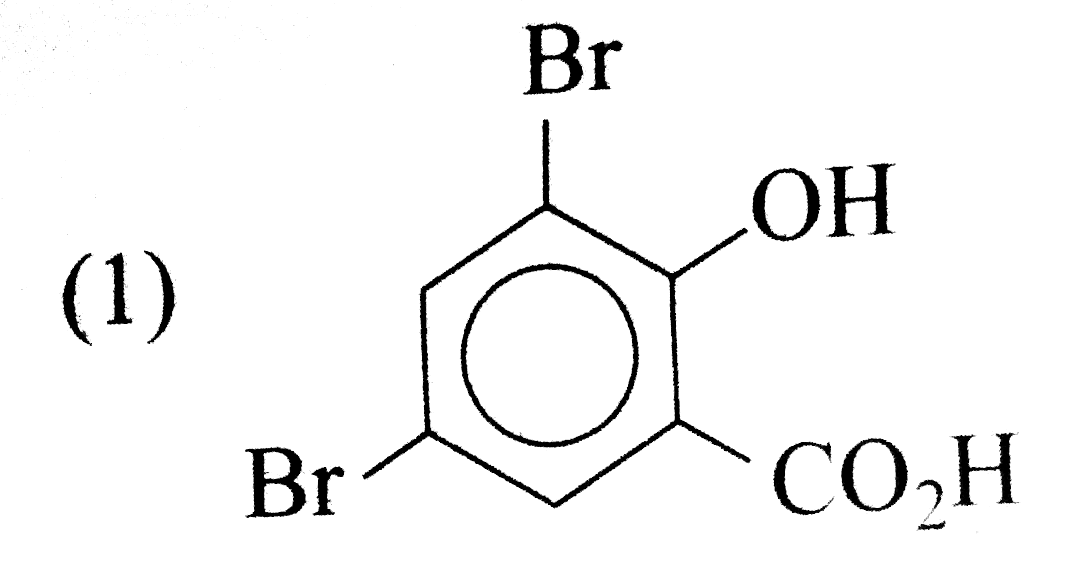
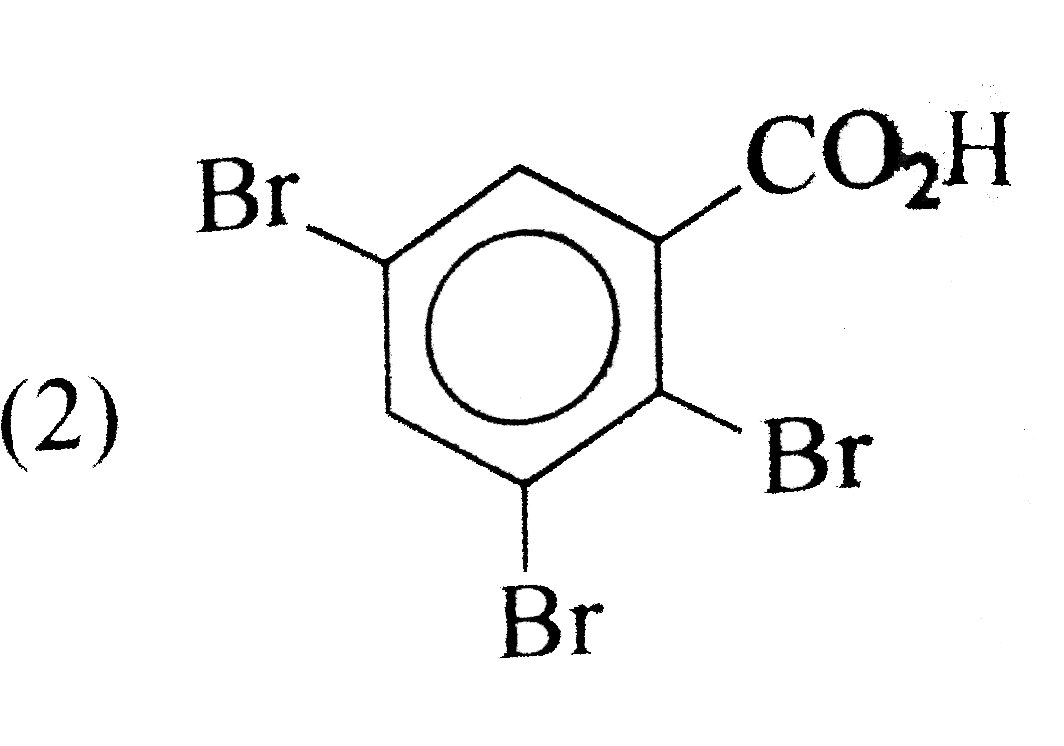
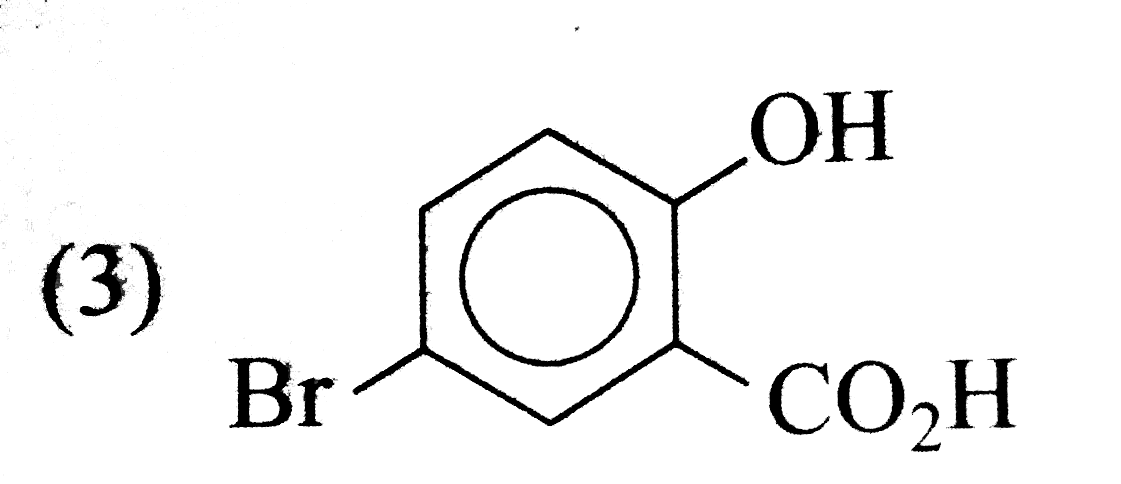
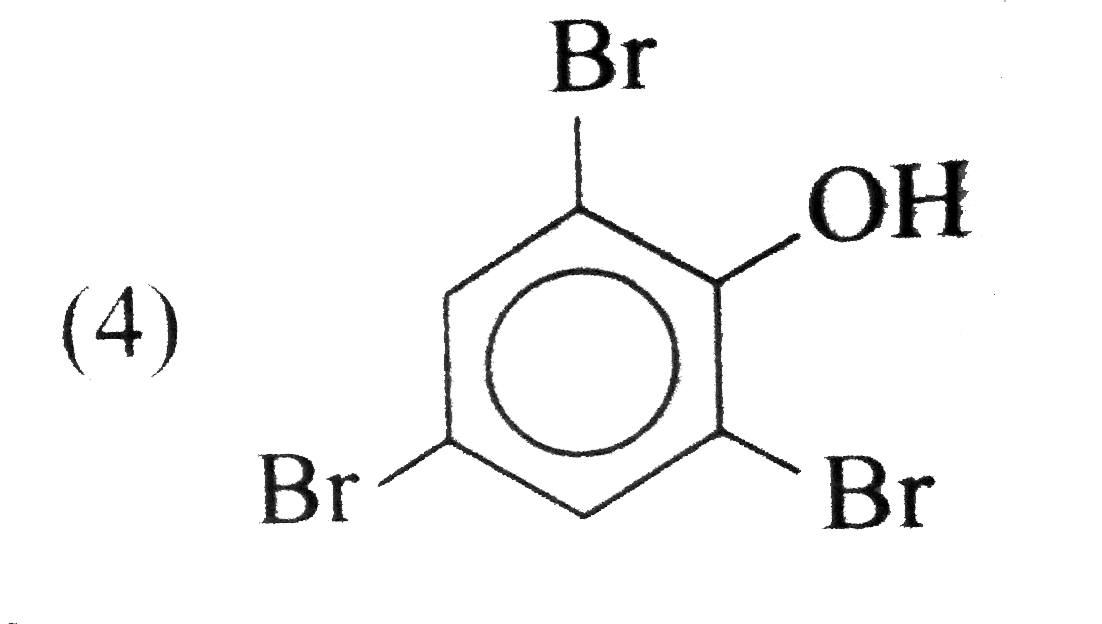
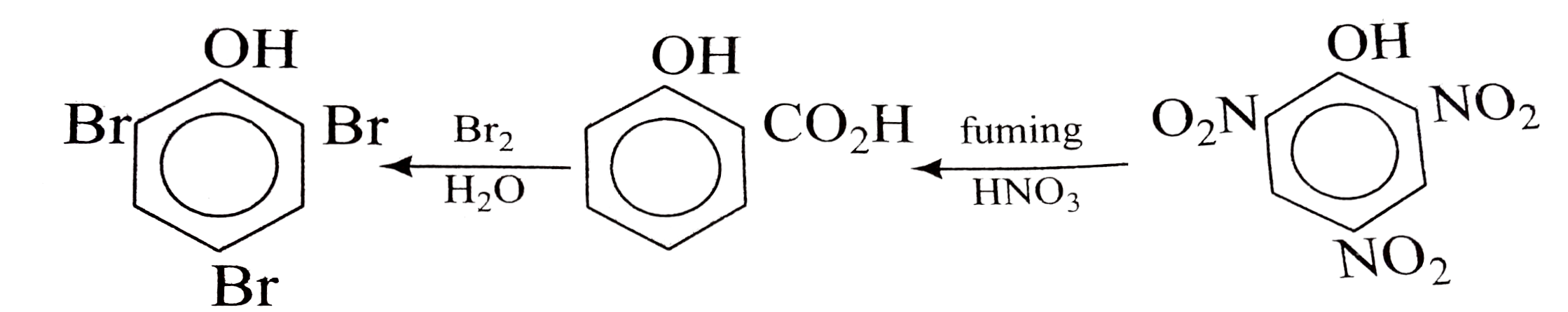
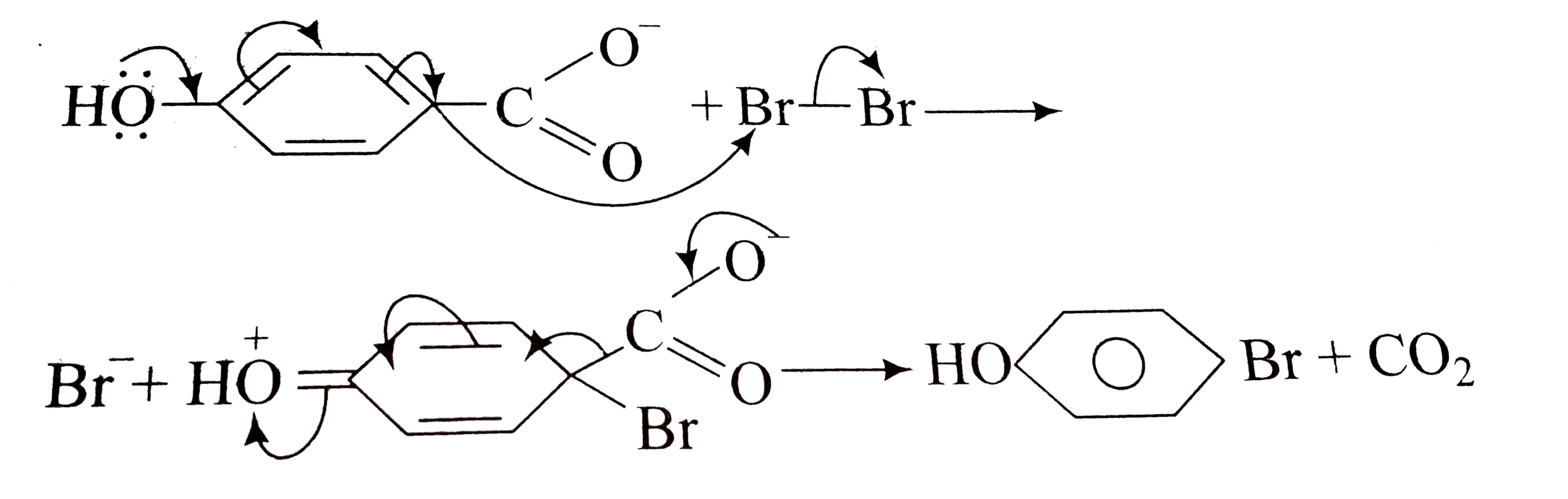
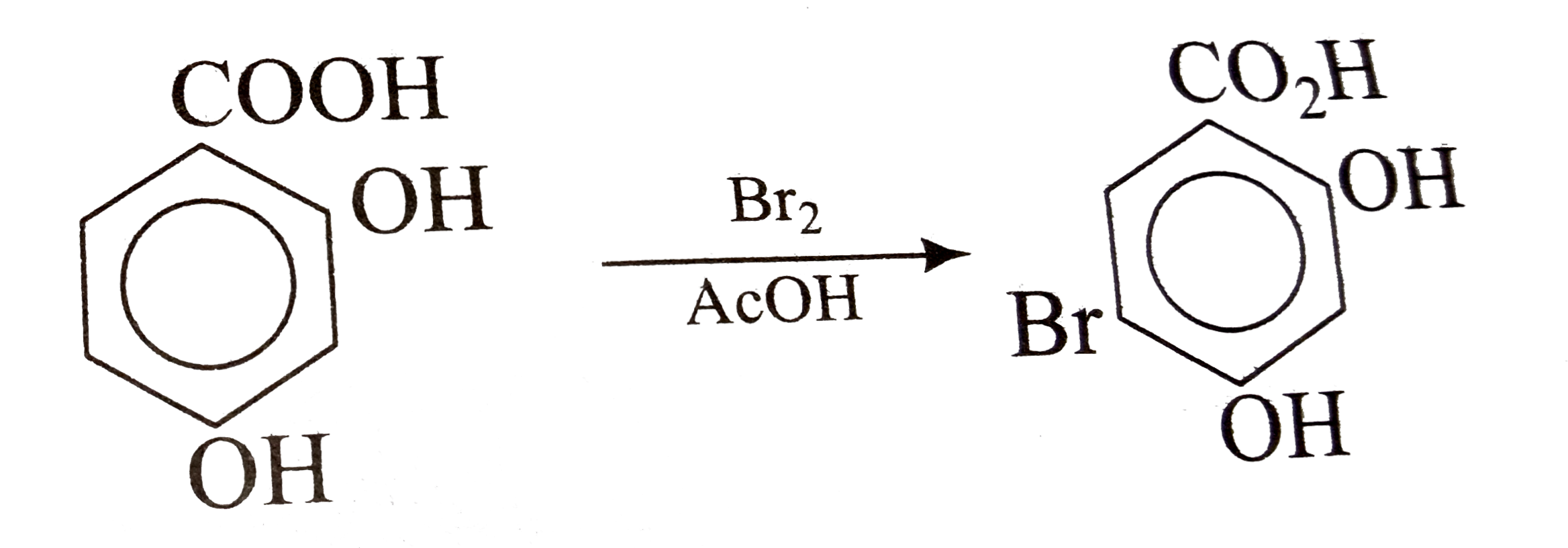A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-PHENOLS (IV)
- Phenolic methyl ethers are obtained in excellent yield by the action o...
Text Solution
|
- The nitration of phenol (and of aniline) is acelerated by the presence...
Text Solution
|
- The aciton of bromine water (excess) on salicylic acid results in the ...
Text Solution
|
- Which of the following is formed when picric acid is reduced with sodi...
Text Solution
|
- When sodium phenoxide is heated with CO(2) under a presence of 100 atm...
Text Solution
|
- Salicylaldehyde is treated with alkaline hydrogen peroxide and subsequ...
Text Solution
|
- Consider the following reaction Which of the following is (are) f...
Text Solution
|
- Compound A,C(7)H(8)O, is insoluble in water, dilute HCl, and aquenous ...
Text Solution
|
- The major product of the reaction is
Text Solution
|
- Schotten Baumann reaction is
Text Solution
|