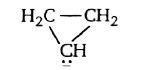
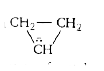A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL-CO-ORDINATION COMPOUNDS-LEVEL 3 (PASSAGE TYPE)
- The magnetic property, dipole moment, plane of symmetry, colour and ab...
Text Solution
|
- Ligands are broadly classified into two classes classical and non-clas...
Text Solution
|
- Ligands are broadly classified into two classes classical and non-clas...
Text Solution
|
- Ligands are broadly classified into two classes classical and non-clas...
Text Solution
|
- An isomerr of the complex CoBrCl(2)(en)(2)(H(2)O), on reaction with co...
Text Solution
|
- An isomerr of the complex CoBrCl(2)(en)(2)(H(2)O), on reaction with co...
Text Solution
|
- Crystal field theory provides correct electronic distribution of centr...
Text Solution
|
- Crystal field theory provides correct electronic distribution of centr...
Text Solution
|
- Crystal field theory provides correct electronic distribution of centr...
Text Solution
|
- Two important physical evidence support the synergic bonding in non-cl...
Text Solution
|
- Two important physical evidences supporting the synergic bonding in no...
Text Solution
|
- Two important physical evidences supporting the synergic bonding in no...
Text Solution
|
- Complex compounds that have the same molecular formula but have differ...
Text Solution
|
- Complex compounds that have the same molecular formula but have differ...
Text Solution
|
- Complex compounds that have the same molecular formula but have differ...
Text Solution
|
- A complex compound of chromium chromium contains five NH(3) molecules,...
Text Solution
|
- A complex compound of chromium chromium contains five NH(3) molecules,...
Text Solution
|
- A complex compound of chromium chromium contains five NH(3) molecules,...
Text Solution
|
- According to C.F.T, attraction between the central metal ion and ligan...
Text Solution
|
- According to C.F.T, attraction between the central metal ion and ligan...
Text Solution
|