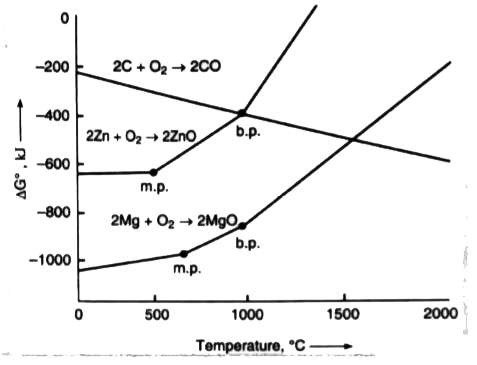
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
METALLURGY
VK JAISWAL|Exercise Level 3 (Passive 3)|6 VideosMETALLURGY
VK JAISWAL|Exercise One OR MORE ANSWERS IS/ ARE CORRECT|75 VideosMETALLURGY
VK JAISWAL|Exercise Level 3 (Passive 1)|5 VideosENVIRONMENTAL CHEMISTRY
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|14 Videosp-BLOCK ELEMENTS
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|36 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-METALLURGY-Level 3 (Passive 2)
- The ellingham diagram for zinc, magnesium and carbon coverting into co...
Text Solution
|
- The ellingham diagram for zinc, magnesium and carbon coverting into co...
Text Solution
|
- The ellingham diagram for zinc, magnesium and carbon coverting into co...
Text Solution
|
- The ellingham diagram for zinc, magnesium and carbon coverting into co...
Text Solution
|
- The ellingham diagram for zinc, magnesium and carbon coverting into co...
Text Solution
|