Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise HIGHER ORDER THINKING SKILLS (HOTS PROBLEMS)|3 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise VALUE BASED QUESTIONS WITH ANSWERS|4 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise ADDITIONAL QUESTIONS (LONG ANSWER QUESTIONS)|6 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|29 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ALCOHOLS, PHENOLS AND ETHERS-HIGHER ORDER THINKING SKILLS (QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTION)
- PCl(5) reactws with ethanol to form chloroethane. However, with phenol...
Text Solution
|
- Alcohols donot react with NaBr but when H.SO, is added they form alkyl...
Text Solution
|
- Although both allyl alccohol and 1-propanol are primary alcohols, the...
Text Solution
|
- 2,2-Dimethyloxirane can be cleaved by acid (H^(o+)). Write the mechani...
Text Solution
|
- Write down the structure of the product of the following reactions:
Text Solution
|
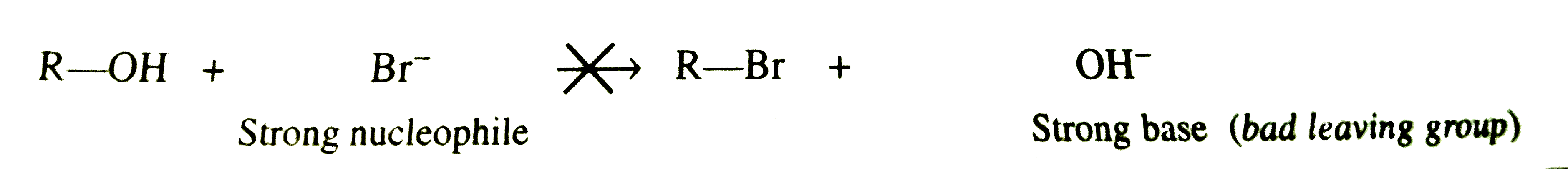
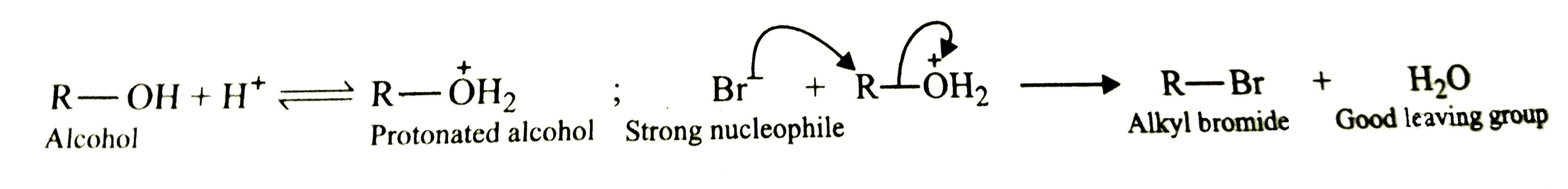 .
.