Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise ADDITIONAL QUESTIONS (VERY SHORT ANSWER QUESTIONS)|70 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise ADDITIONAL QUESTIONS (SHORT ANSWER QUESTIONS)|74 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (ASSERTION AND REASON TYPE QUESTIONS)|5 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATIONS.|29 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (LONG ANSWER QUESTIONS)
- An alkene 'A' (molecular formula C(5)H(10)) on ozonolysis gives a mixt...
Text Solution
|
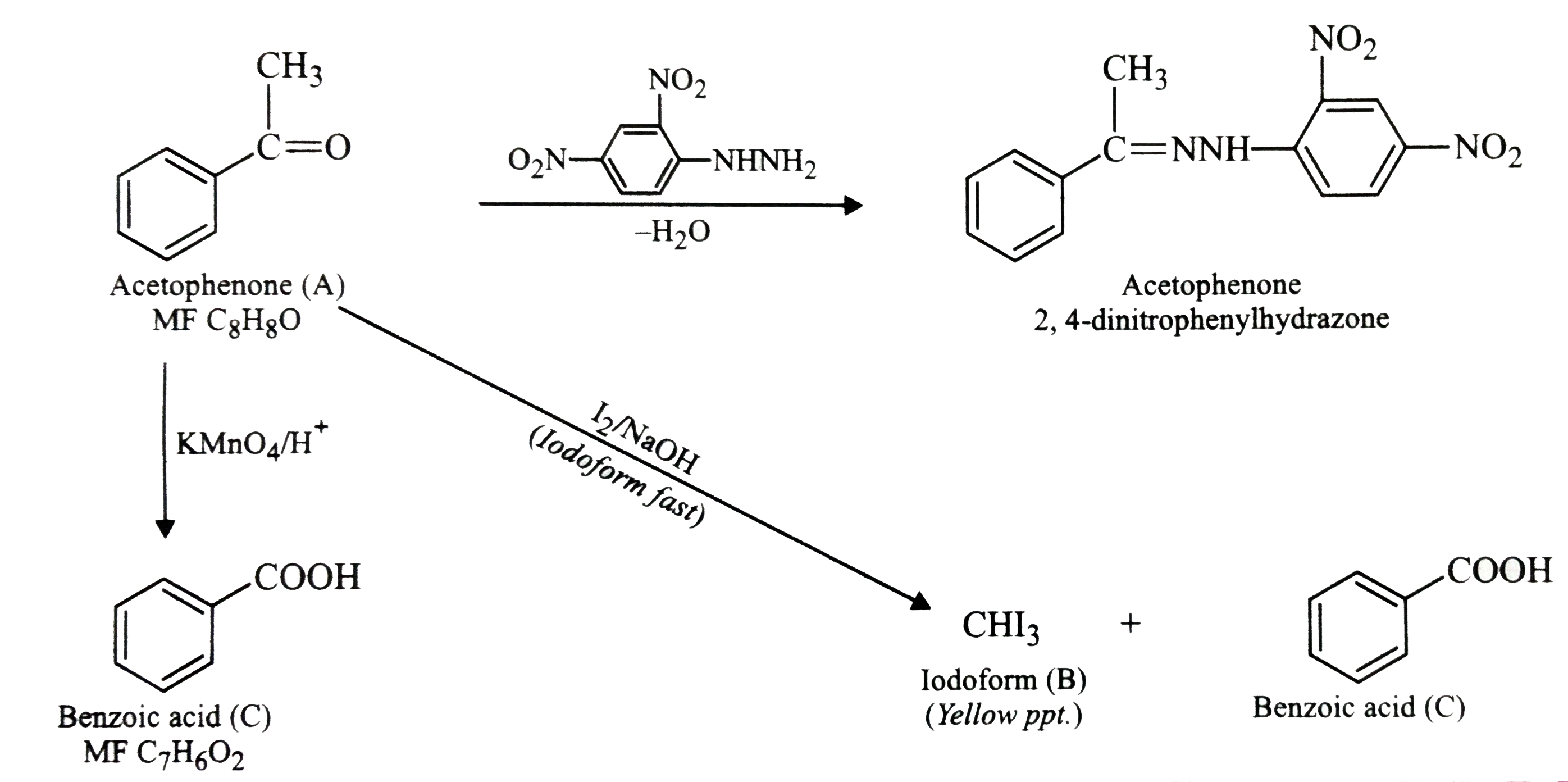
- An aromatic compound 'A' (Molecular formula C(8)H(8)O)) gives positive...
Text Solution
|
- Write down functional isomers of a carbonyl compound with molecular fo...
Text Solution
|
- When liquid 'A' is treated with a freshly prepared ammoniacal silver n...
Text Solution
|