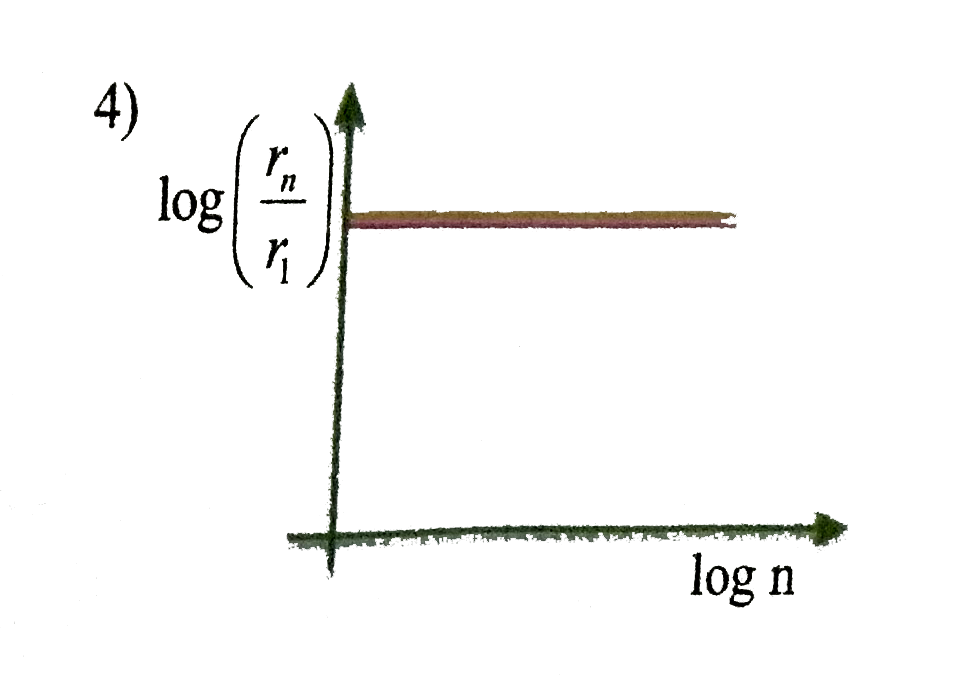
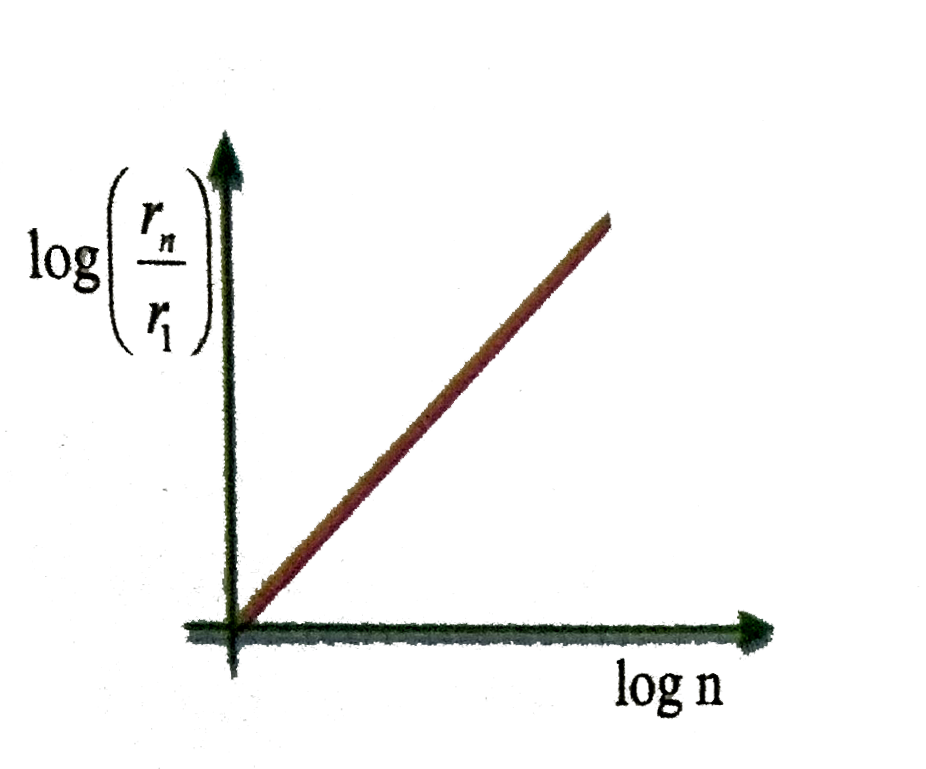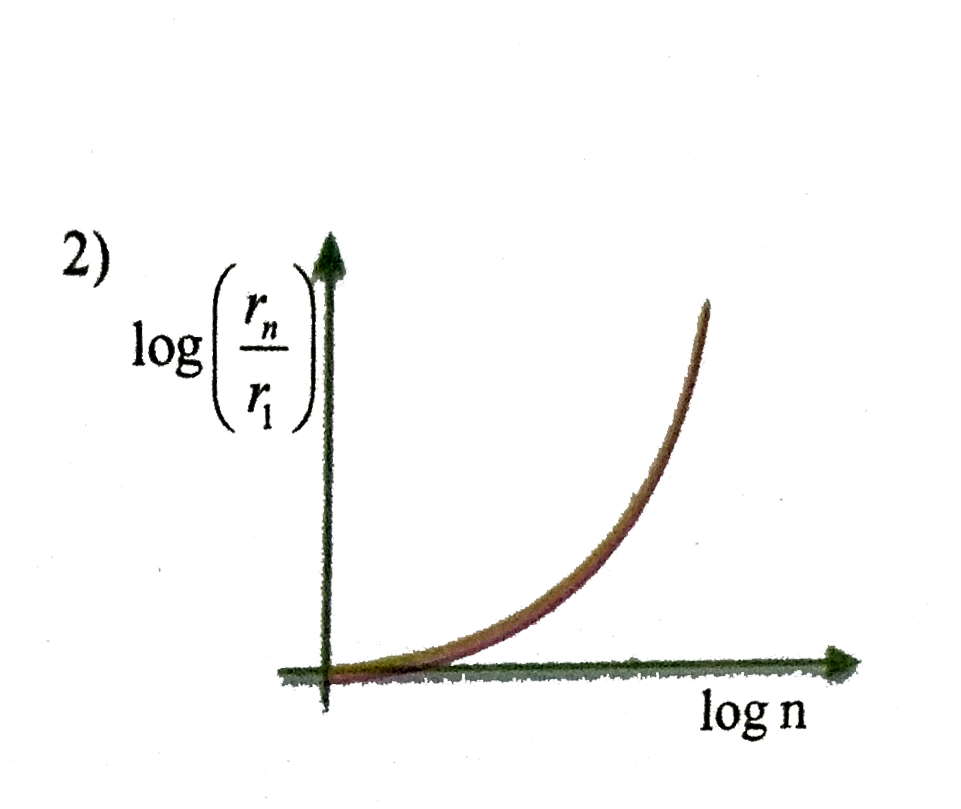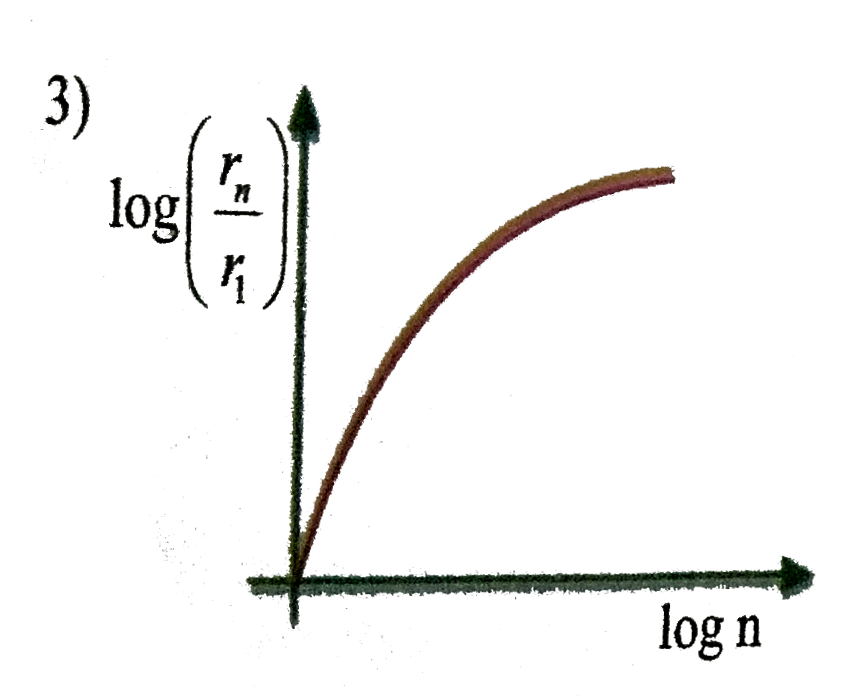A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ATOMIC PHYSICS-LEVEL-III
- If elements of quantum number greater than n were not allowed , the nu...
Text Solution
|
- Magnetic field at the center (at nucleus) of the hydrogen like atom ("...
Text Solution
|
- In hydrogen atom, the radius of n^(th) Bohr orbit is V(n). The graph B...
Text Solution
|
- In hydrogen atom, the area enclosed by n^(th) orbit is A(n). The graph...
Text Solution
|
- If we assume only gravitational attraction between protect and electro...
Text Solution
|
- A particle of charge equal to that of an electron - e, and mass 208 ti...
Text Solution
|
- A particle known as mu meson has a charge equal to that of no electron...
Text Solution
|
- A particle of charge equal to that of an electron - e, and mass 208 ti...
Text Solution
|
- photoelectron are emitted when 4000A^(0) radiation is incident on a su...
Text Solution
|
- phtoelectron are emitted when 4000A^(0) radiation is incident on a sur...
Text Solution
|
- phtoelectron are emitted when 4000A^(0) radiation is incident on a sur...
Text Solution
|
- A sample of hydrogen gas in its ground state is irradiated with photon...
Text Solution
|
- A sample of hydrogen gas in its ground state is irradiated with photon...
Text Solution
|
- A sample of hydrogen gas in its ground state is irradiated with photon...
Text Solution
|
- A sample of hydrogen gas in its ground state is irradiated with photon...
Text Solution
|
- A sample of hydrogen gas in its ground state is irrdation with photon ...
Text Solution
|
- A sample of hydrogen gas in its ground state is irrdation with photon ...
Text Solution
|
- A sample of hydrogen gas in its ground state is irrdation with photon ...
Text Solution
|
- A sample of hydrogen gas in its ground state is irrdation with photon ...
Text Solution
|
- Which of the following statement is true regarding Bohr's model of hyd...
Text Solution
|