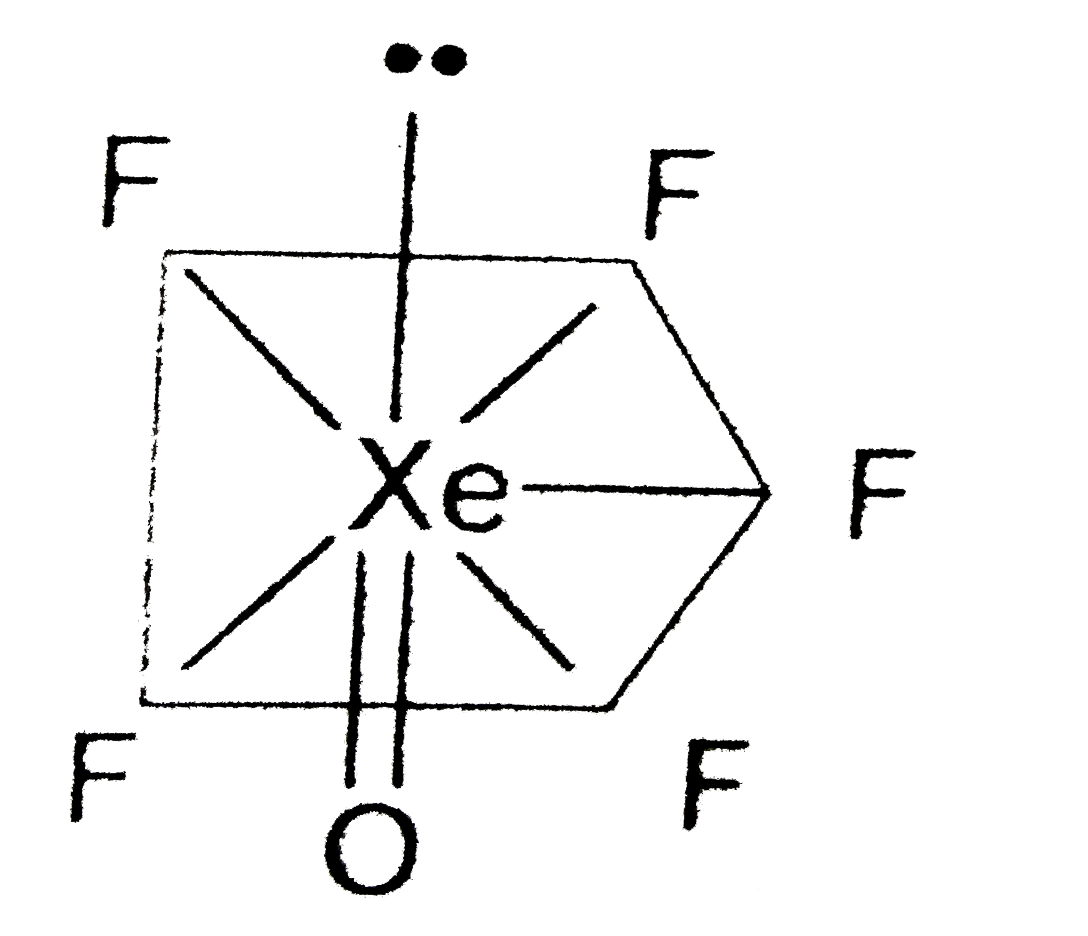
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FIITJEE-TEST PAPERS-CHEMISTRY
- Ferrocene is a compound of
Text Solution
|
- Which of the following statement is incorrect
Text Solution
|
- According to VSEPR model, the shape of [XeOF(5)]^(-) is
Text Solution
|
- Identify the statement(s) which is not correct with respect to surface...
Text Solution
|
- How many dischloride cyclopentanes (including stereoisomers) are obtai...
Text Solution
|
- The major product obtained when following substrate is subjected to E2...
Text Solution
|
- the end prodcut of the reaction is
Text Solution
|
- Structure of (A) and (B) respectively are:
Text Solution
|
- An aqueous solution of a metal ion (A) on reaction with Kl gives a bla...
Text Solution
|
- Which of the following compounds consists of P-P linkage?
Text Solution
|
- Which of the following elements of lanthanide series have highest tend...
Text Solution
|
- The specific conductance of a saturated solution of silver bromide is ...
Text Solution
|
- Which of the following is diamagnetic ?
Text Solution
|
- A certain amount of reducing agent reduces x mole of KMnO(4) & y mole ...
Text Solution
|
- The activation energies of two reactions are E(1) & E(2) with E(1)gtE(...
Text Solution
|
- The wavelength of the first Lyman lines of hydrogen He^(+) and Li^(2+)...
Text Solution
|
- Number of electrons having m(1)=0 for sodium atom is
Text Solution
|
- The following two reactions: i. PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) ...
Text Solution
|
- The following curve shows the change of pH during the course of titrat...
Text Solution
|
- The minimum mass of NaBr which should be added in 200 ml of 0.0004M-Ag...
Text Solution
|