Text Solution
Verified by Experts
Topper's Solved these Questions
P BLOCK ELEMENTS
RESONANCE|Exercise Exercise 1 part-1|14 VideosP BLOCK ELEMENTS
RESONANCE|Exercise Exercise 1 part-2|32 VideosP BLOCK ELEMENTS
RESONANCE|Exercise ALP PART OBJECTIVE|12 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise STAGE-II|1 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-P BLOCK ELEMENTS-B.L.E
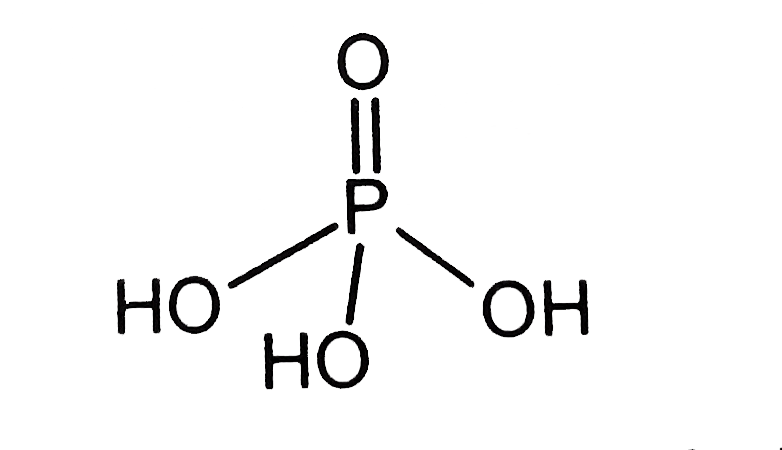
- What is the basicity of H(3)PO(4) ?
Text Solution
|
- Solid phosphorus pentachloride behaves as an ionic compound. Explain
Text Solution
|
- Phosphorus shows greater tendency for catenation than nitrogen. Why ?
Text Solution
|
- Give the chemical reaction to support that +5 oxidation state of Bi is...
Text Solution
|
- Bi(V) and Sb(V) which may be a stronger oxidizing agent and why ?
Text Solution
|
- Bi(2)O(3) is treated stronger oxidizing agent, write a balanced equati...
Text Solution
|
- Sulphur disappears when boiled with an aqueous solution of sodium sulp...
Text Solution
|
- Write one chemical reaction to show that conc. H(2)SO(4) can act as an...
Text Solution
|
- Write the chemical equation for the following : Ca(3)(PO(4))(2) + Si...
Text Solution
|
- Write balanced equation when NH(3) is dissolved in water.
Text Solution
|
- What is the oxidation number of phosphorus in H(3)PO(2) molecule ?
Text Solution
|
- Why is N(2) less reactive at room temperature ?
Text Solution
|
- List the important sources of sulphur.
Text Solution
|
- Why does O(3) act as a powerful oxidizing agent?
Text Solution
|
- How is ozone estimated?
Text Solution
|
- The HNH angle value is higher than HPH, HAsH and HSbH angles.Why ?
Text Solution
|
- Nitrogen exists as diatomic molecule and phosphorus as P(4). Why ?
Text Solution
|
- Among the hydrides of group 16, water shows unusual physical propertie...
Text Solution
|
- Draw the structures of (i)PCl(5)(s), (ii)SO(3)^(2-)
Text Solution
|
- On being slowly passed through water, PH(3) forms bubbles but NH(3) di...
Text Solution
|