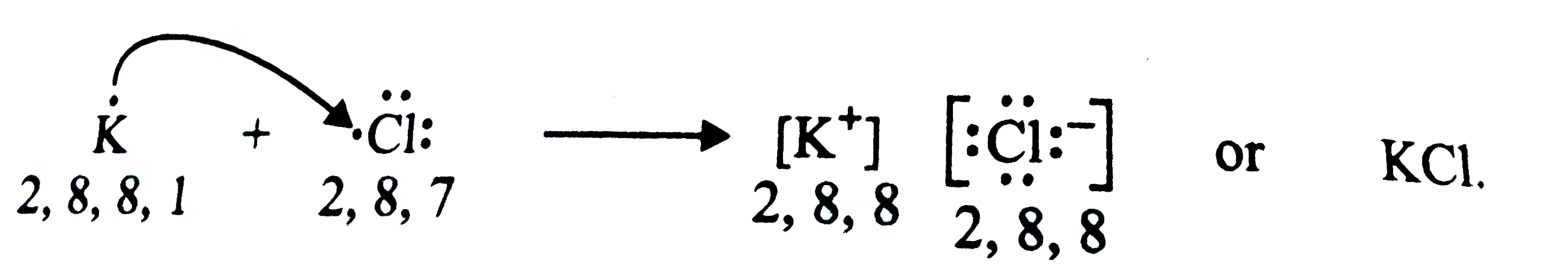Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Test Your Grip Multiple choice questions I|15 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Test Your Grip (Fill in the Blanks) II|15 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Problems For Practice|27 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise Curiosity Questions|2 VideosENVIRONMENTAL CHEMISTRY
PRADEEP|Exercise COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance (VI.ASSERTION-REASON) Type II|6 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -Advanced Problems
- From the following elements: ""4Be, ""9F, ""19K, ""20Ca (i) ...
Text Solution
|
- The following table shows the position of six elements A,B,C,D,...
Text Solution
|
- Four elements A ,B ,C and D along with their electonic configuratio...
Text Solution
|
- A part of the Periodic Table is given below : Now answser the ...
Text Solution
|
- An element X has same number of electrons in the first and the ...
Text Solution
|