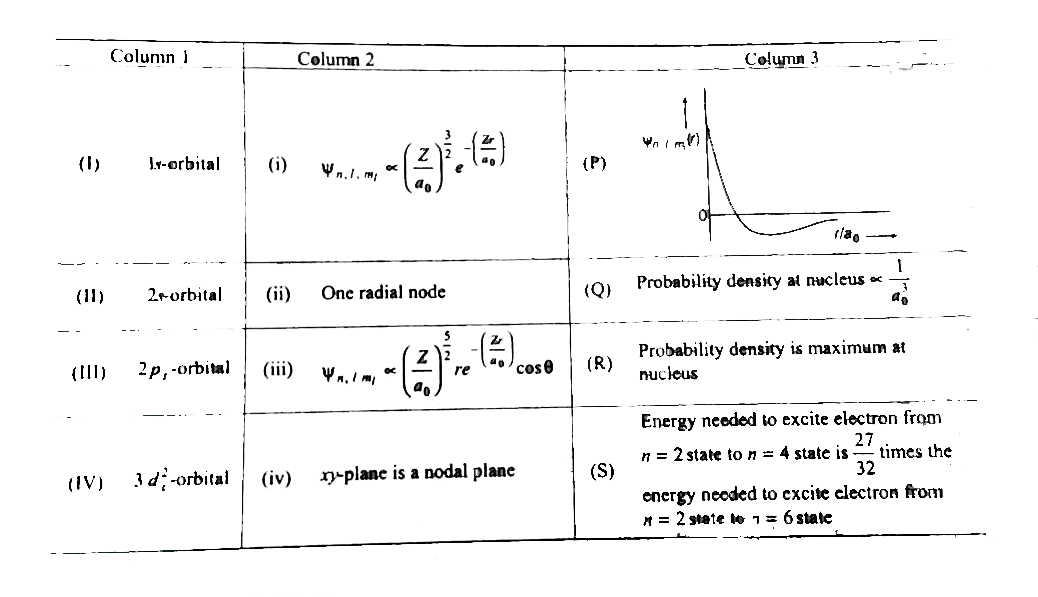A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IIT-JEE PREVIOUS YEAR (CHEMISTRY)-ATOMIC STRUCTURE-Match the Columns
- The wave function, psi(n), l, m(l) is a mathematical function whose va...
Text Solution
|
- The wave function, psi(n), l, m(l) is a mathematical function whose va...
Text Solution
|
- The wave function, psi(n), l, m(l) is a mathematical function whose va...
Text Solution
|
- Match the entires in Column I with the correctly related quantum numbe...
Text Solution
|