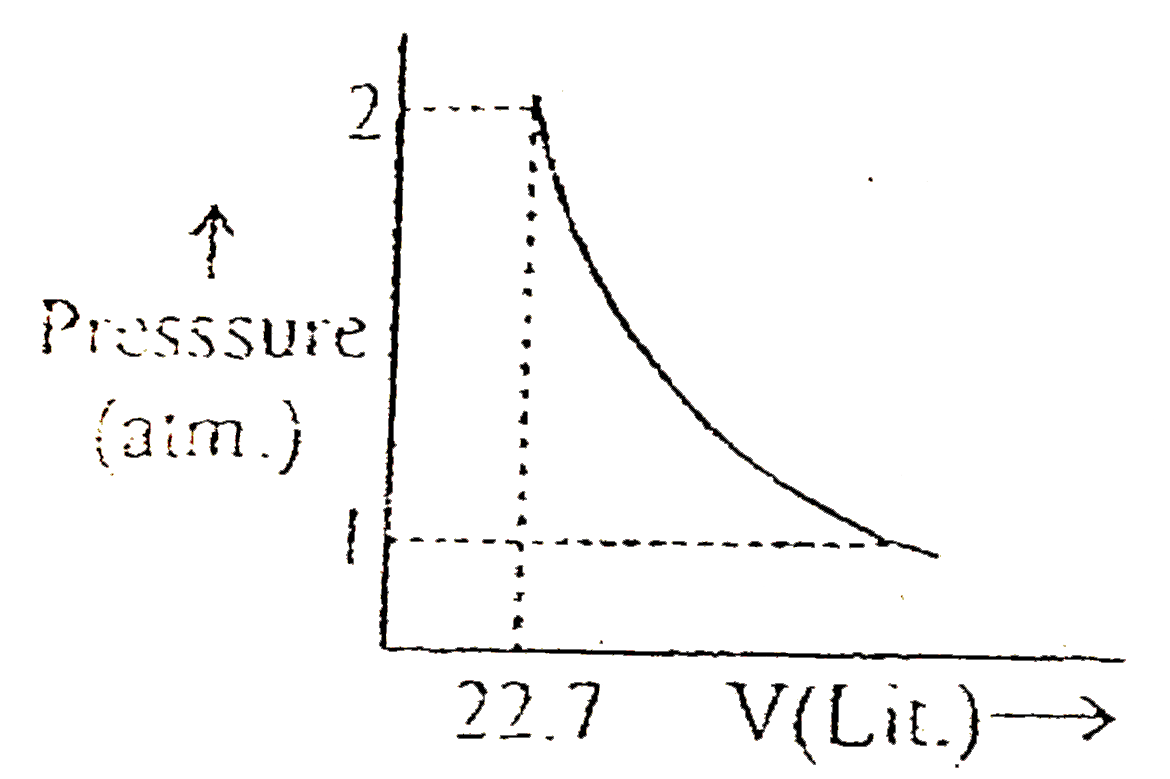A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-TEST PAPER-Exercise (chemistry)
- An aqueous solution contains 980 mg of H(2)SO(4), 3.01xx10^(21) molec...
Text Solution
|
- A metallic oxide contains 60% metal by mass.The vapour density of vola...
Text Solution
|
- One mole of an ideal and monoatomic gas is expanded reversibly and iso...
Text Solution
|
- Predict which of the following reactions have positive entropy changes...
Text Solution
|
- Which of the following relation is correct for the calculation of work...
Text Solution
|
- The enthalpies of neutralization to two weak acids HA and HB with NaOH...
Text Solution
|
- At 0^(@)C , ice and water are in equilibrium and DeltaS and DeltaG for...
Text Solution
|
- In the followin electronic configuration, ns^(2)(n-1)d^(0-1)(n-2)f^(...
Text Solution
|
- Which of the following electronic confirgration in the outermost shell...
Text Solution
|
- The radius of isoelectronic series :-
Text Solution
|
- In which of the following compounds cation and anion size ratio is min...
Text Solution
|
- Set of elements having one electron in their valence shell is :-
Text Solution
|
- A neutral atom (A) is converted to (A) by the following process Aove...
Text Solution
|
- Which of the following processes involves absorption of energy?
Text Solution
|
- The electron affinity of
Text Solution
|
- Select correct diagram about the acidic strength of oxides :-
Text Solution
|
- In which of the following pairs both the molecules are non existing?
Text Solution
|
- Substance which is not required in Solvay process :-
Text Solution
|
- Which of the following does not produce any gaseous product when react...
Text Solution
|
- CaCO(3)overset(Delta)rarrResidue+gas. Residue when dissolves in water,...
Text Solution
|