Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-KINETIC THEORY OF GASES-Exercise-4
- Two moles of an ideal monoatomic gas is taken through a cycle ABCA as ...
Text Solution
|
- A monoatomic ideal gas, initially at temperature T1, is enclosed in a ...
Text Solution
|
- Starting with the same initial conditions, an ideal gas expands from v...
Text Solution
|
- P-V plots for two gases during adiabatic processes are shown in the fi...
Text Solution
|
- In a given process on an ideal gas, dW=0 and dQlt0. Then for the gas
Text Solution
|
- A monoatomic ideal gas of two moles is taken through a cyclic process ...
Text Solution
|
- An ideal gas is taken through the cycle AtoBtoCtoA, as shown in the fi...
Text Solution
|
- Which of the following graphs correctly represents the variation of be...
Text Solution
|
- A cubical box of side 1 m contains helium gas (atomic weight 4) at a p...
Text Solution
|
- The PT diagram for an ideal gas is shown in the figure, where AC is an...
Text Solution
|
- An insulated container containing monoatomic gas of molar mass s is mo...
Text Solution
|
- An ideal gas expands isothermally from volume V(1) to V(2) and is then...
Text Solution
|
- The piston cylinder arrangement shon contains a diatomic gas at temper...
Text Solution
|
- An ideal gas is filled in a closed rigid and thermally insulated conta...
Text Solution
|
- When temperature of a gas is 20^@C and pressure is changed from p(1)= ...
Text Solution
|
- A cylinder of mass 1kg is given heat of 20,000J at atmospheric pressur...
Text Solution
|
- Match the following for the given process: {:(column 1,Column2),((A)...
Text Solution
|
- The piston I s now pulled out slowly and held at a diatance 2L from th...
Text Solution
|
- While the piston is at a distance 2L from the top, the hole at the top...
Text Solution
|
- The piston is taken completely out of the cylinder. The hole at the to...
Text Solution
|
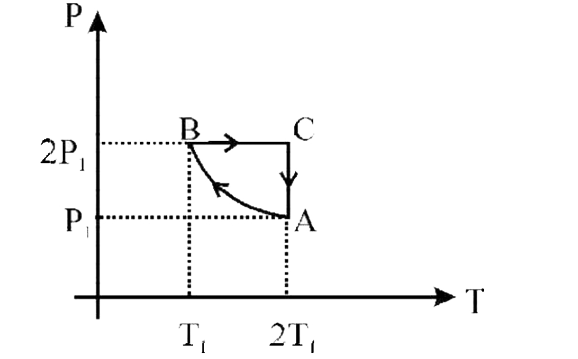
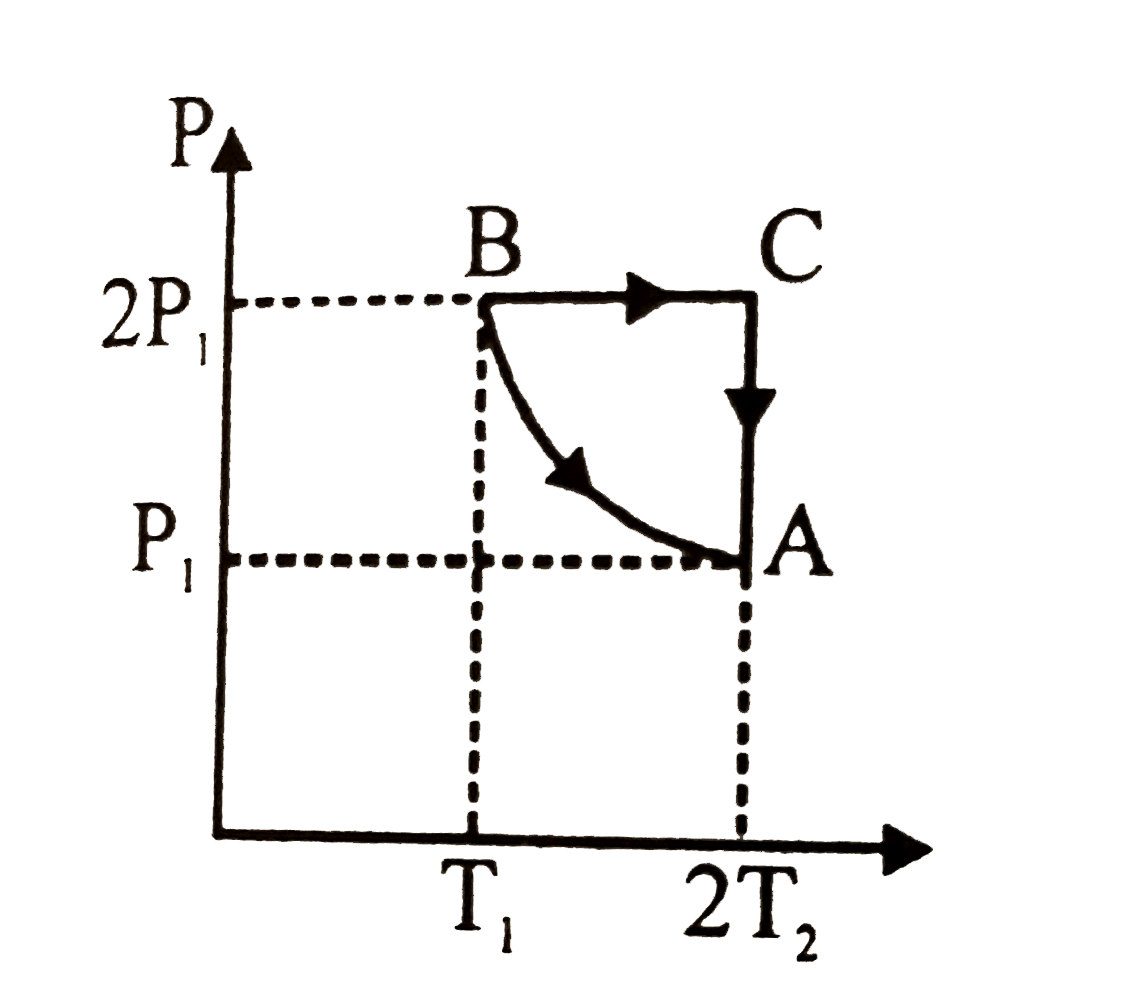 Number of moles, n `= 2,T_(1)=300K`
Number of moles, n `= 2,T_(1)=300K`