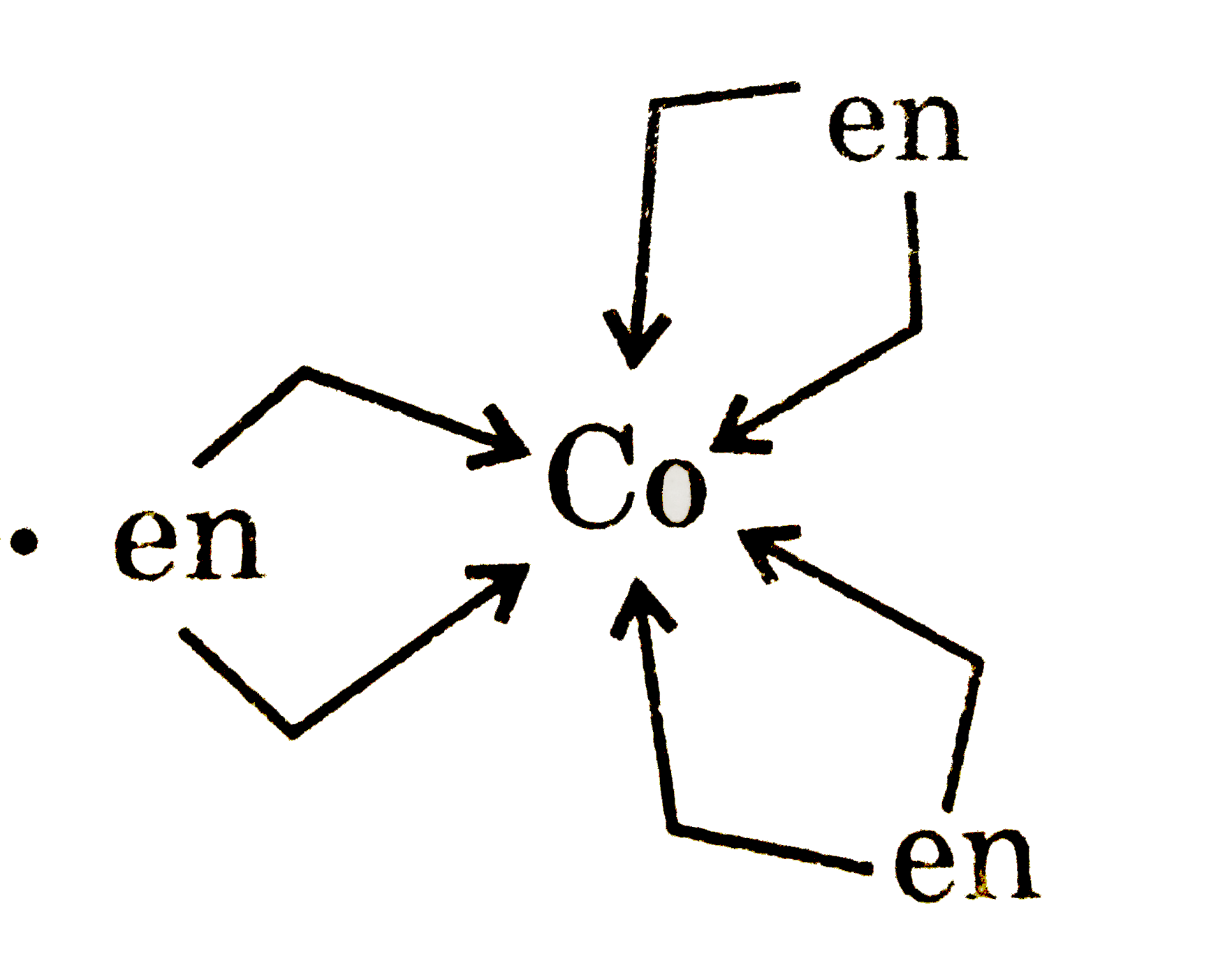
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
GRB PUBLICATION|Exercise MULTIPLE OBJECTIVE TYPE QUESTION|130 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise COMPREHENSION 1|3 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise G. Organometallic Compounds, Synergic Bonding|52 VideosCHEMICAL KINETICS
GRB PUBLICATION|Exercise Subjective Type|63 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-COORDINATION COMPOUNDS-REASONING TYPE
- Statement-1: [Co(NH(3))(5)SO(4)]Br" and "[Co(NH(3))(5)Br]SO(4) are ion...
Text Solution
|
- Statement-1: In OC-M-CO if one CO is replaced by better pi-a c c e p t...
Text Solution
|
- Statement-1: [Co(en)(3)]^(3+) show stereoisomerism. Statement-2: EAN...
Text Solution
|
- Statement-1: In Zeise's salt C-C bond order regarding ethylene molecul...
Text Solution
|
- Statement-1: [V(CO)(6)] cannot act as oxidising agent. Statement-2: ...
Text Solution
|
- Statement-2: Hund's rule violates in [Co(CN)(6)]^(3-) complex ion. S...
Text Solution
|
- Assertion: Complex ion [Co(NH(3))(6)]^(2+) is readily oxidized to [Co(...
Text Solution
|
- Statement-1: Aqueous CuSO(4) solution on reaction with excess of aqueo...
Text Solution
|
- Statement-1: [Ti(H(2)O)(6)]^(3+) is violet in colour while Ti^(3+) is ...
Text Solution
|
- Statement-1: Toxic metal ions are removed by the chelating ligands. ...
Text Solution
|
- Assertion (A) [Cr(H(2)O(6))]Cl(2) and [Fe(H(2)O)(6)]Cl(2) are reducing...
Text Solution
|
- Assertion (A) Linkage isomerism arises in coordination compounds conta...
Text Solution
|
- Statement-1: Complexes of MX(6)" and "MX(5)L type (X and L are unident...
Text Solution
|
- Assertion (A) [Fe(CN)(6)]^(3-) ion shows magnetic moment corresponding...
Text Solution
|
- Statement-1: [Cu(NH(3))(4)]^(2+)" and "[Cu(CN(4))]^(3-) ions are colou...
Text Solution
|
- Statement-1: NH(2)NH(2) athough possesses two electron pairs for donat...
Text Solution
|
- Statement-1: In the reaction [CoCl(2)(NH(3))(4)]^(+)+Cl^(-) to [CoCl(3...
Text Solution
|
- Statement-1: The complex CoBr(3)4NH(3).2H(2)O has molar conductivity c...
Text Solution
|
- Statement-1: The correct distribution of 3d electrons of chromium in t...
Text Solution
|
- Statement-1: The correct order for the wave length of absorption in th...
Text Solution
|