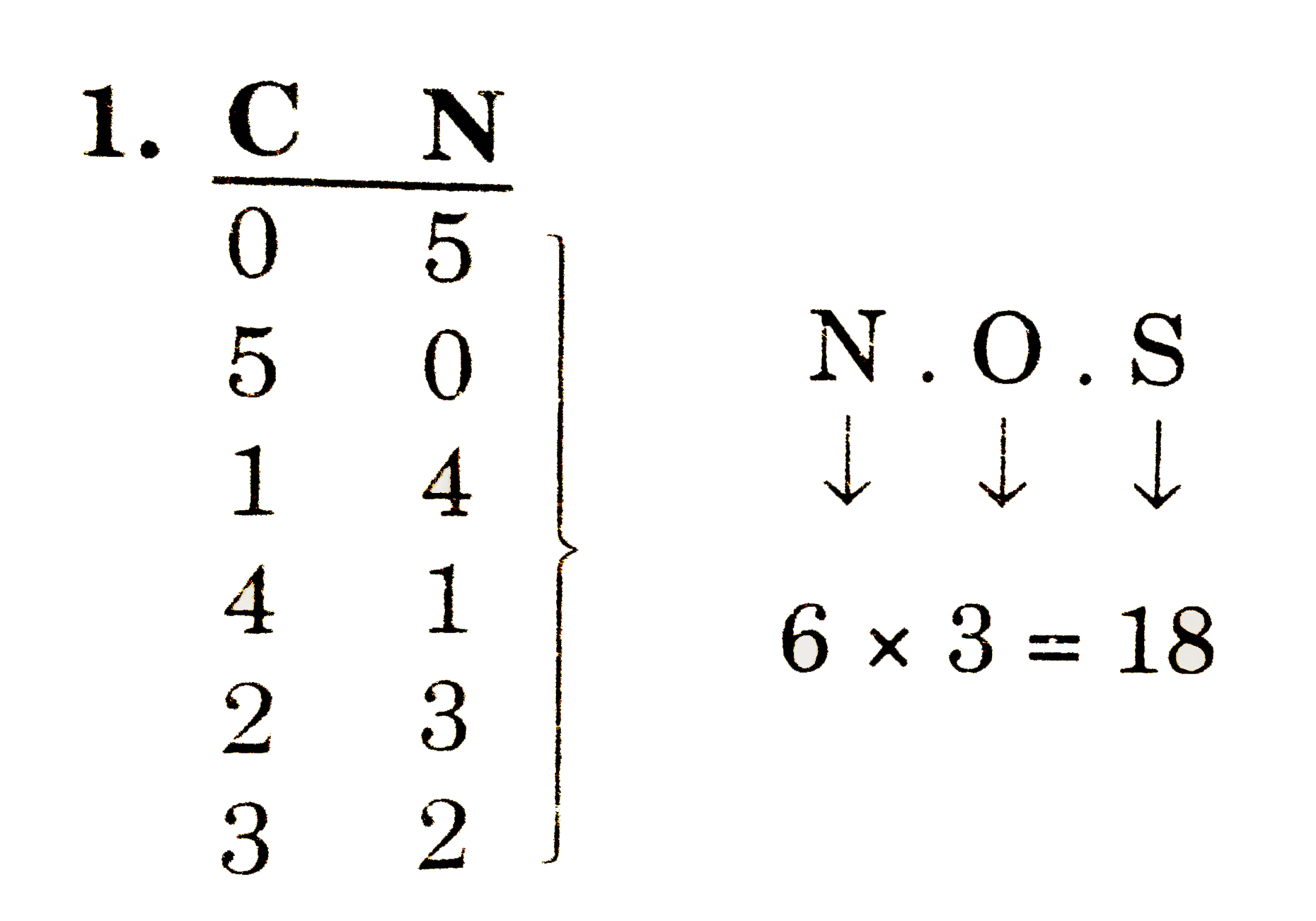A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
GRB PUBLICATION|Exercise COMPREHENSION 9|2 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise COMPREHENSION 10|3 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise COMPREHENSION 7|2 VideosCHEMICAL KINETICS
GRB PUBLICATION|Exercise Subjective Type|63 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 Videos
Similar Questions
Explore conceptually related problems