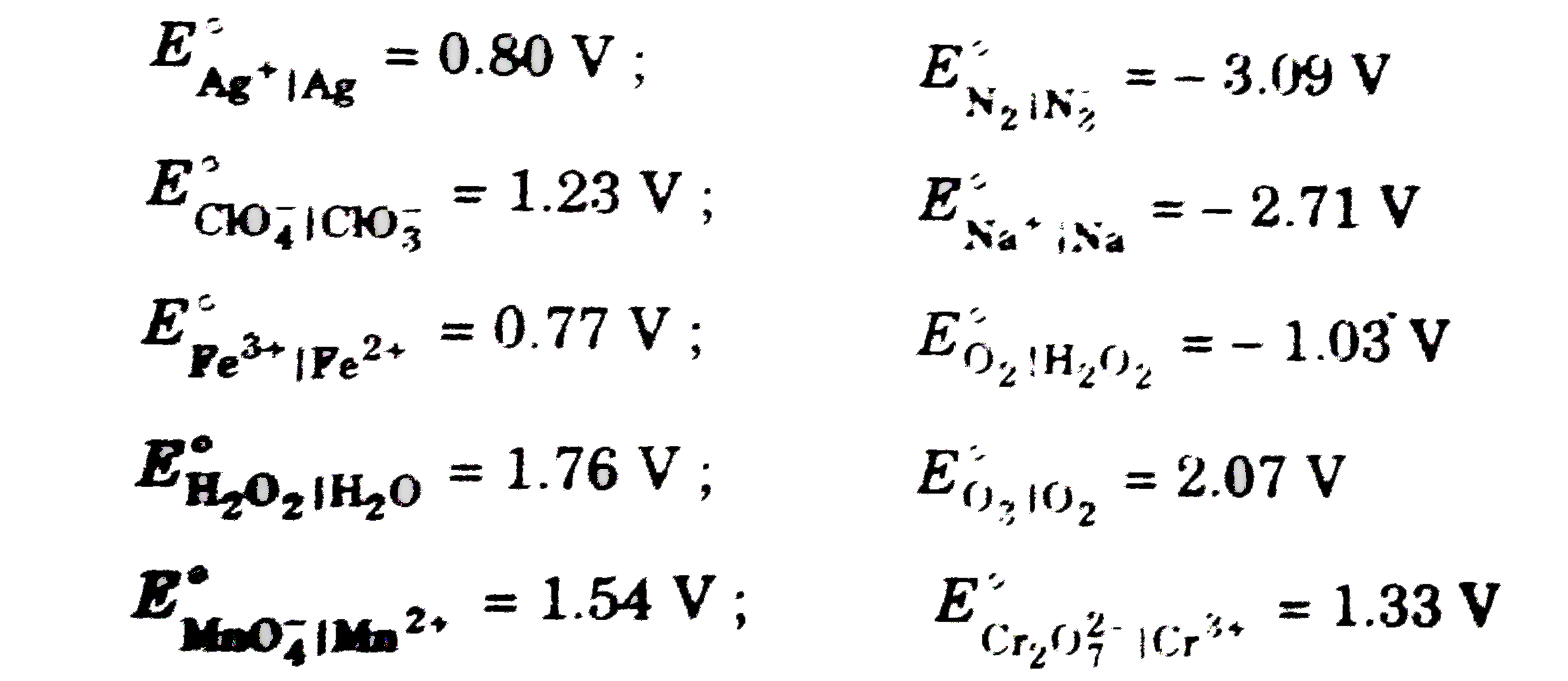A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTY
GRB PUBLICATION|Exercise Match the column type|16 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Subjective type|39 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Multiple objective type|33 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 VideosENVIRONMENTAL CHEMISTRY
GRB PUBLICATION|Exercise Straight objective type|40 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ELECTROCHEMISTY-Comprehension type
- Standard reduction potentials (SRP) for different systems can be used ...
Text Solution
|
- Standard reduction potentials (SRP) for different systems can be used ...
Text Solution
|
- Standard reduction potentials (SRP) for different systems can be used ...
Text Solution
|
- [Given: E(Zn^(2+)|Zn)^(@)=-.0.76V K(f)[Cu(NH(3))(4)]^(+2)=4xx10^(11)...
Text Solution
|
- Given: E(Zn^(+2)//Zn)^(@) =- 0.76V E(Cu^(+2)//Cu)^(@) = +0.34V K...
Text Solution
|
- [Given: E(Zn^(2+)|Zn)^(@)=-.0.76V K(f)[Cu(NH(3))(4)]^(+2)=4xx10^(11)...
Text Solution
|
- The molar conductance of NaCl varies with the concentration as shown i...
Text Solution
|
- The molar conductance of NaCl varies with the concentration as shown i...
Text Solution
|
- The molar conductance of NaCl vauies with the concentration as shown i...
Text Solution
|
- A fuel cell is a cell that is continuously supplied with an oxidant an...
Text Solution
|
- A fuel cell is a cell that is continuously supplied with an oxidant an...
Text Solution
|
- A fuel cell is a cell that is continuously supplied with an oxidant an...
Text Solution
|
- A fuel cell is a cell that is continuously supplied with an oxidant an...
Text Solution
|
- A fuel cell is a cell that is continuously supplied with an oxidant an...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- A sample of water from a large swimming pool has a resistance of 10000...
Text Solution
|
- A sample of water from a large swimming pool has a resistance of 10000...
Text Solution
|
- A sample of water from a large swimming pool has a resistance of 10000...
Text Solution
|