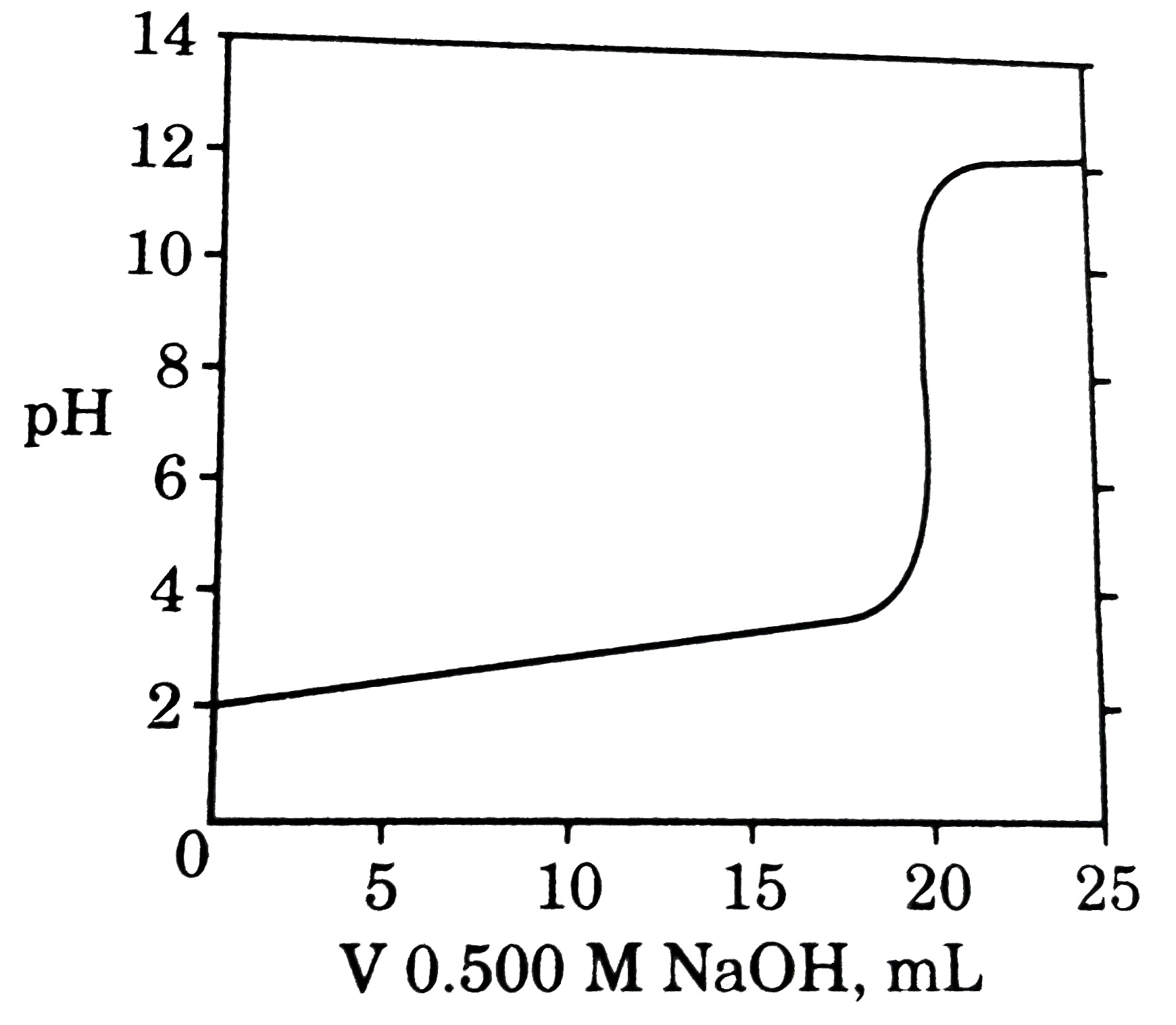
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise G. Chemical Equilibrium|1 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Chemical Equilibrium|36 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise F. Ionic Equilibrium|1 VideosF-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective type|7 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Previous years jee questions|28 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GRAPHICAL INTERPRETATION-Ionic Equilibrium
- A sample of 100 mL of a solution of a weak monoprotic acid of unknown ...
Text Solution
|
- A 0.100M aqueous solution of H(2)SeO(3) is titrated with 1.000M NaOH s...
Text Solution
|
- A buffer solution is prepared by mixing 'a' moles of CH(3)COONa and 'b...
Text Solution
|
- Which is/are correct statements ? (P) In any strong acid's solution,...
Text Solution
|
- Which is/are correct statement ? (P) When 100 mL of 0.1M NaCN solut...
Text Solution
|
- A 25.0 mL sample of waste water is obtained to analyze for Pb^(2+) ion...
Text Solution
|
- The curve represents the titration of a weak monoprotic acid. Ovar wha...
Text Solution
|
- The curve represents the titration of :
Text Solution
|