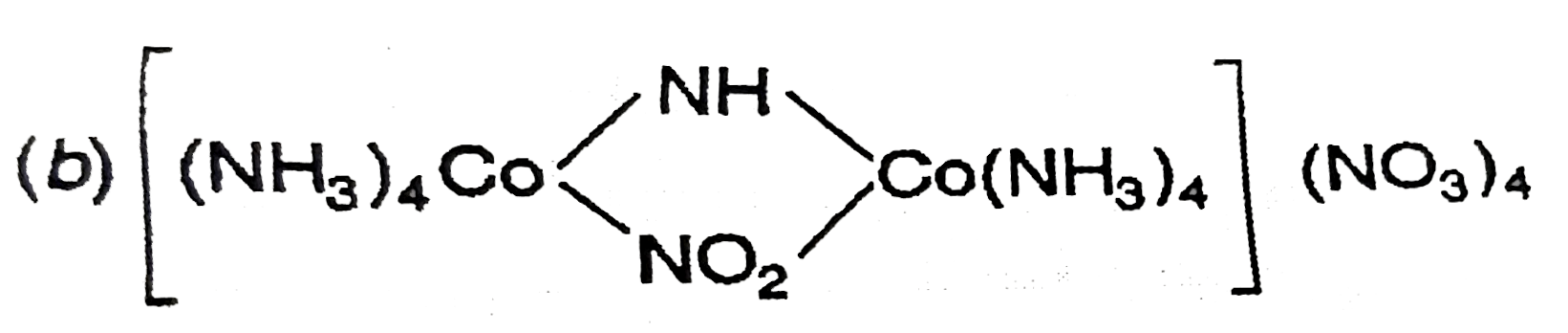
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-COORDINATION COMPOUNDS-BITSAT Archives
- the oxidation state of cobalt in
Text Solution
|
- Which of the following will not optilcal isomers ?
Text Solution
|
- The magnitude of Delta(0) will be highest in which of the following co...
Text Solution
|
- Which of the following is an outer d-orbital or high spin complex ?
Text Solution
|
- Which of the following is correct IUPAC name for K(2)[Co(CN)(2)O(2)(O)...
Text Solution
|
- Ferrocene is an examle of
Text Solution
|
- [Co(NH(3))(5)'] Br and ]Co(NH(3))(5) Br] SO(4) is a pair of ...... Iso...
Text Solution
|
- The crysal field splitting energy for octahedral (Delta(0)) and tetrah...
Text Solution
|