A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-ELECTROCHEMISTRY-BITSAT Archives
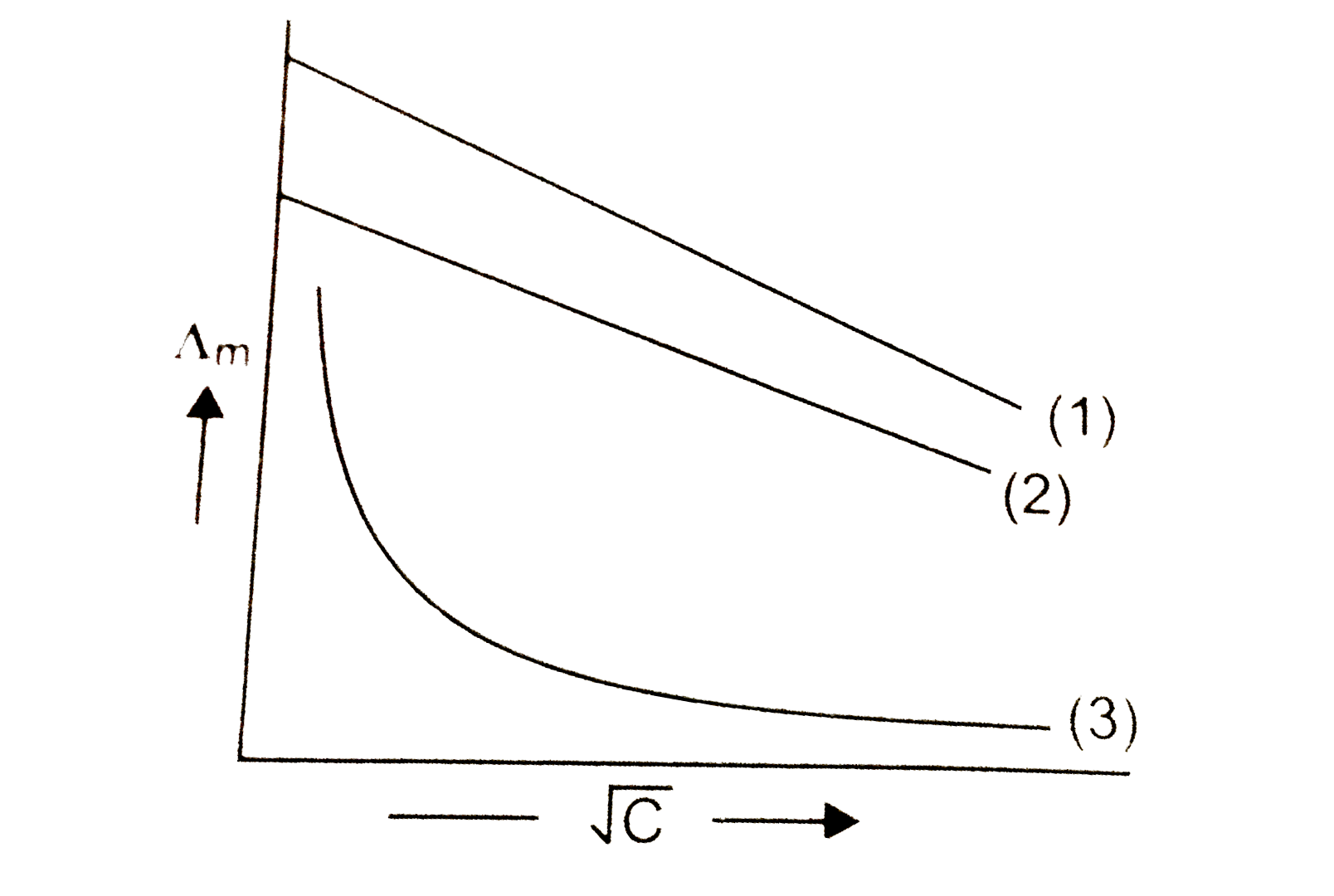
- Molar conductance Lamda(m) is plotted against sqrt(C) (mol "litre"^(-1...
Text Solution
|
- A reaction, Cu^(2+)+2e^(-) rarr Cu is given. For this reaction, graph ...
Text Solution
|
- If E(Fe^(3+)//Fe)^(@) and E(Fe^(2+)//Fe)^(@) are -0.36 V and 0.439 V r...
Text Solution
|
- The molar conducatance of Ba^(2+) and Cl^(-) are 127 and 76 ohm^(-1) c...
Text Solution
|
- The equilibrium constant (K) for the reaction Cu(s)+2Ag^(+) (aq) rar...
Text Solution
|
- E^(@) for Fe//Fe^(2+) is +0.44 V and E^(@) for Cu//Cu^(2+) is -0.32 V....
Text Solution
|
- A curent of 0.5 A when passed through AgNO(3) solution for 193 s depos...
Text Solution
|
- When an aqueous solution of sodium chloride is electrolysed using plat...
Text Solution
|
- When same quantity of electricuty is passed through aqueous AgNO(3) an...
Text Solution
|
- When electric current is passed through acidified water for 1930 s, 11...
Text Solution
|
- Given, standard electrode potentials Fe^(2+) +2e^(-) rarr Fe, E^(@)=...
Text Solution
|
- The specific conductivity of 0.1 N KCl solution is 0.0129 Omega^(-1) c...
Text Solution
|
- The cathodic reaction of a dry cell is represented as 2MnO(2)(s)+Zn^(2...
Text Solution
|
- The standard potential of the reaction H(2)O + e^(-) rightarrow (1/2...
Text Solution
|