You are given two compound
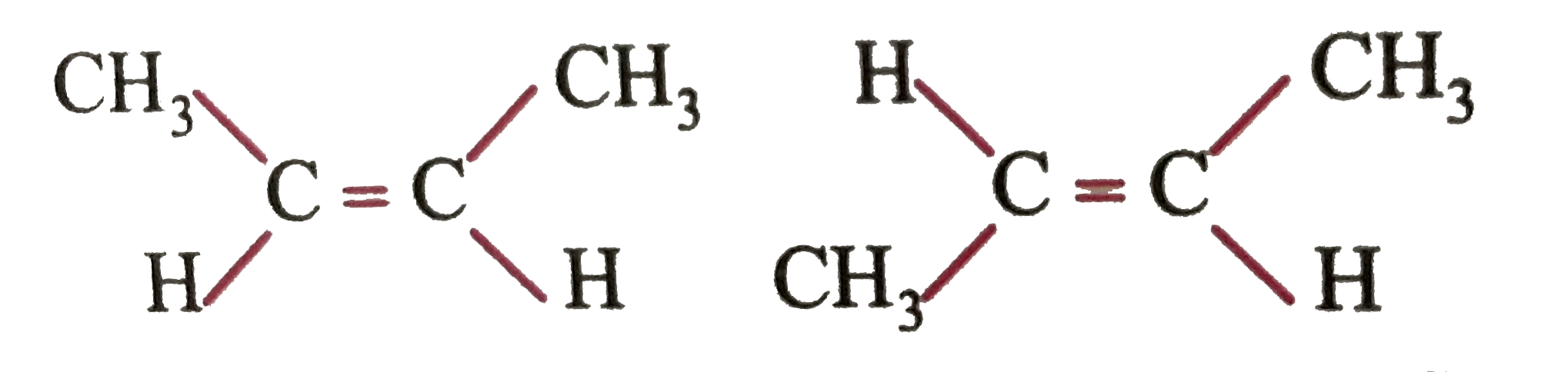
First, you name both of them the same yet, are they really same structure, or they some how different ?Carefully inspection should convince you that they are indeed different. Now, how their physical and chemical properties differ? Generally
(i) cis-isomer has more diplomoment than trans-isomer
(ii) cis-is less stable than trans
(iii) boiling points of cis isomers are than those of trans isomers (remember boiling points are directly related to polarity which is determined by dipolemoment) chemical reactivities of cis and trans also differ from each other.
Which of the following statements best explain(s) the relationship between two isomer of `CH_(3)-CH=CH -CH_(3)`?