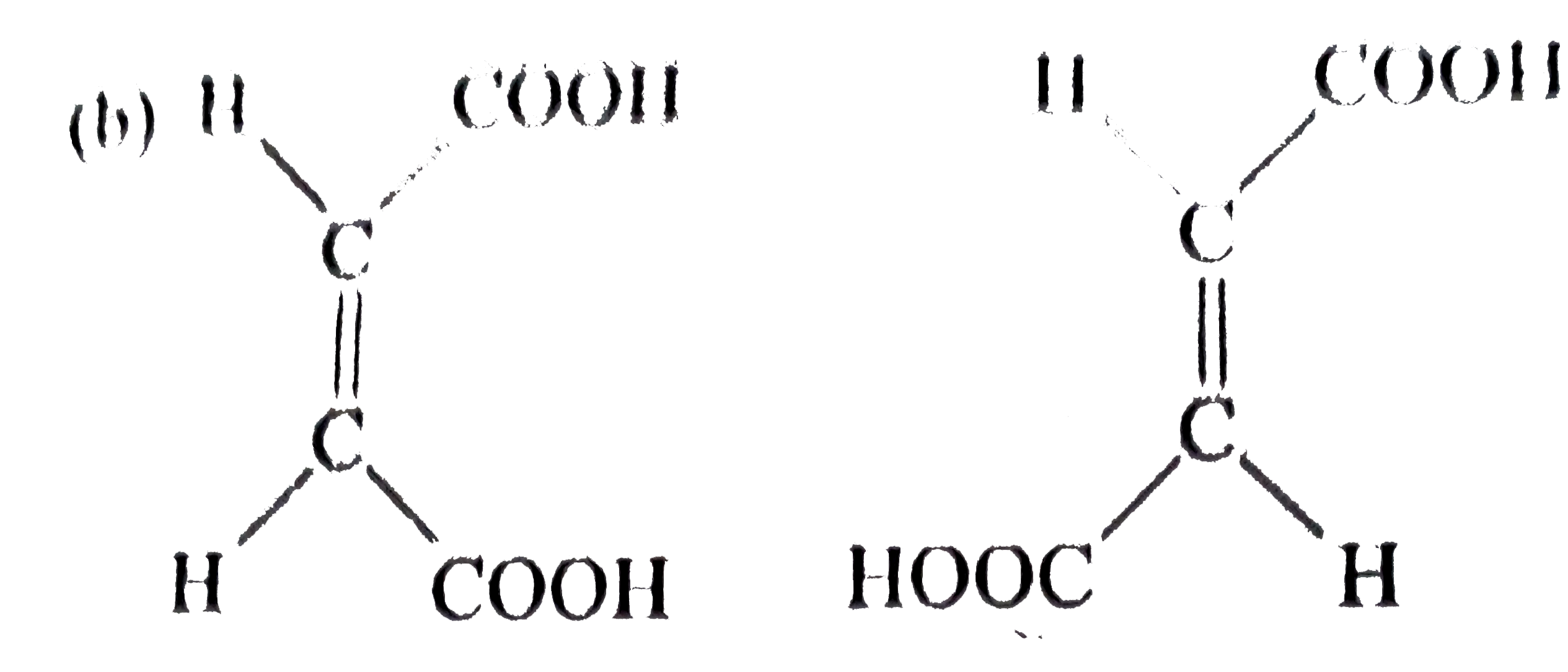
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-PRACTICAL ORGANIC CHEMISTRY-COMPREHENSION_TYPE
- A mixture of carboxyliic acids (X) and (Y) cannot be separated by norm...
Text Solution
|
- A mixture of carboxylic acids (X) and (Y) cannot be separated by norma...
Text Solution
|
- What is the diastereoisomer of (+) - tartaric acid?
Text Solution
|
- To arrive at the molecular formula and at the structural formula the d...
Text Solution
|
- To arrive at the molecular formula and at the structural formula the d...
Text Solution
|
- To arrive at the molecular formula and at the structural formula the d...
Text Solution
|
- Test Q: A compound 'X' was fused with Na metal and the extract gave a ...
Text Solution
|
- Test Q: A compound 'X' was fused with Na metal and the extract gave a ...
Text Solution
|
- Test Q: A compound 'X' was fused with Na metal and the extract gave a ...
Text Solution
|
- Steam distillation is used to purify a compound which is steam princip...
Text Solution
|
- Steam distillation is used to purify a compound which is steam princip...
Text Solution
|
- Steam distillation is used to purify a compound which is steam princip...
Text Solution
|
- Observe the following sequence of reactions P shows geometrical ...
Text Solution
|
- Observe the following sequence of reactions P shows geometrical ...
Text Solution
|
- Observe the following sequence of reactions P shows geometrical ...
Text Solution
|
- An aromatic compound T(C(10)H(10)O(2)) give 2 moles of CHI(3) and comp...
Text Solution
|
- An aromatic compound T(C(10)H(10)O(2)) give 2 moles of CHI(3) and comp...
Text Solution
|
- An aromatic compound T(C(10)H(10)O(2)) give 2 moles of CHI(3) and comp...
Text Solution
|