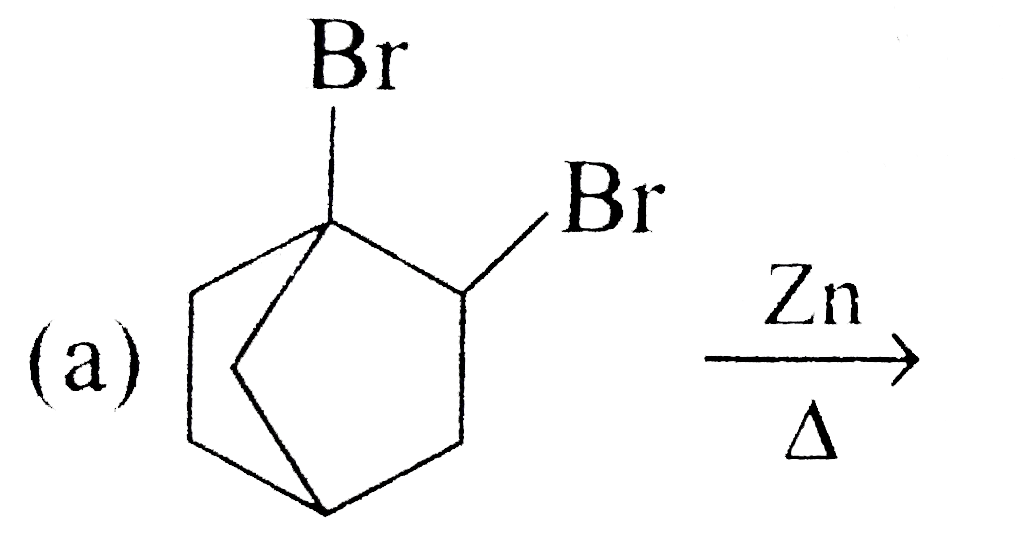
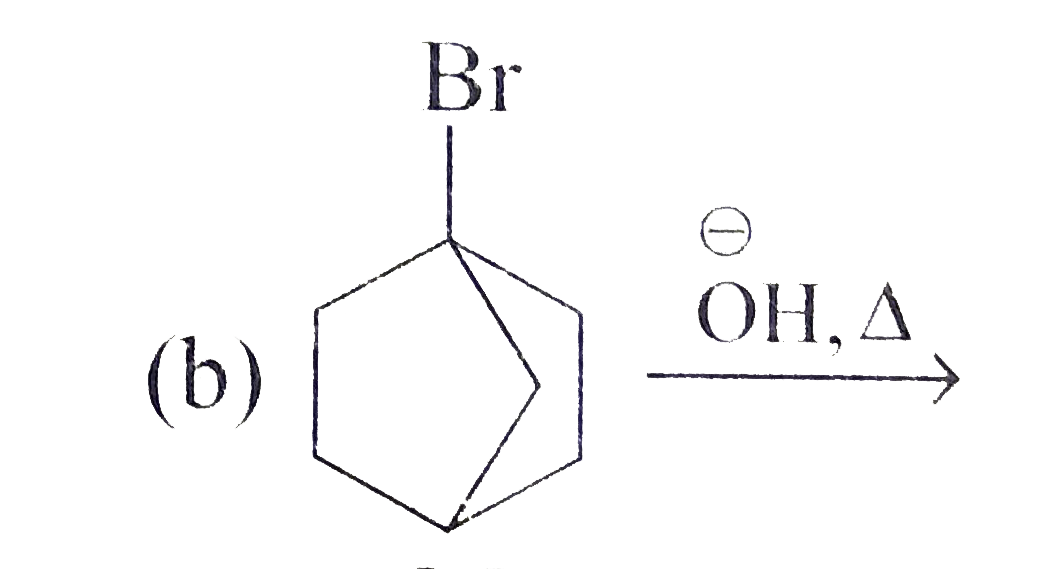
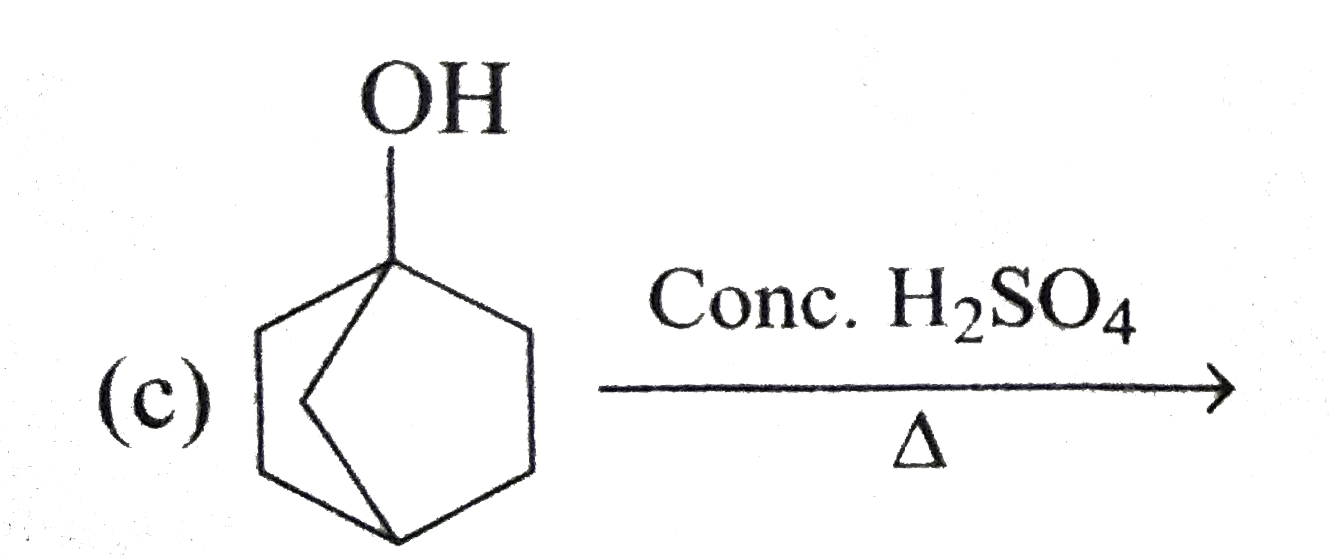
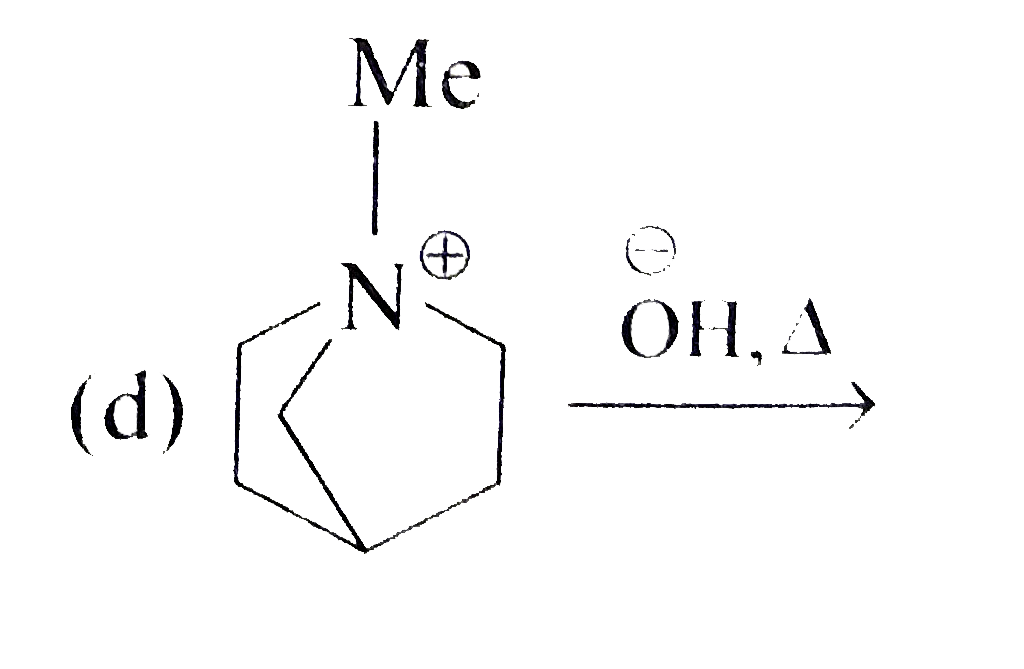
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALIDES
HIMANSHU PANDEY|Exercise Level 2 (Q.126 To Q.150)|25 VideosHALIDES
HIMANSHU PANDEY|Exercise Level 2 (Q.151 To Q.165)|15 VideosHALIDES
HIMANSHU PANDEY|Exercise Level 2 (Q.76 To Q.100)|24 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Subjective Type Problems|19 VideosISOMERISM
HIMANSHU PANDEY|Exercise Subjective Type Problems|11 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-HALIDES-Level 2 (Q.101 To Q.125)
- Major product of the following reaction is :
Text Solution
|
- Major product of the following reaction is :
Text Solution
|
- Complete the following reaction
Text Solution
|
- Which of the following will undergo fastest elimination with alcoholic...
Text Solution
|
- Which of the following is beta-elimination reaction ?
Text Solution
|
- Arrange the following in decreasing order E(2) reaction :
Text Solution
|
- Which of the following reactions will undergo an elimination reaction ...
Text Solution
|
- In the above reaction, maximum Saytzeff product will be obtained where...
Text Solution
|
- In the above reaction (Question no.113) Hofmann product is major when ...
Text Solution
|
- Find the major product of the reaction :
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Identify the major product of the reaction :
Text Solution
|
- Identify the major product Y :
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|