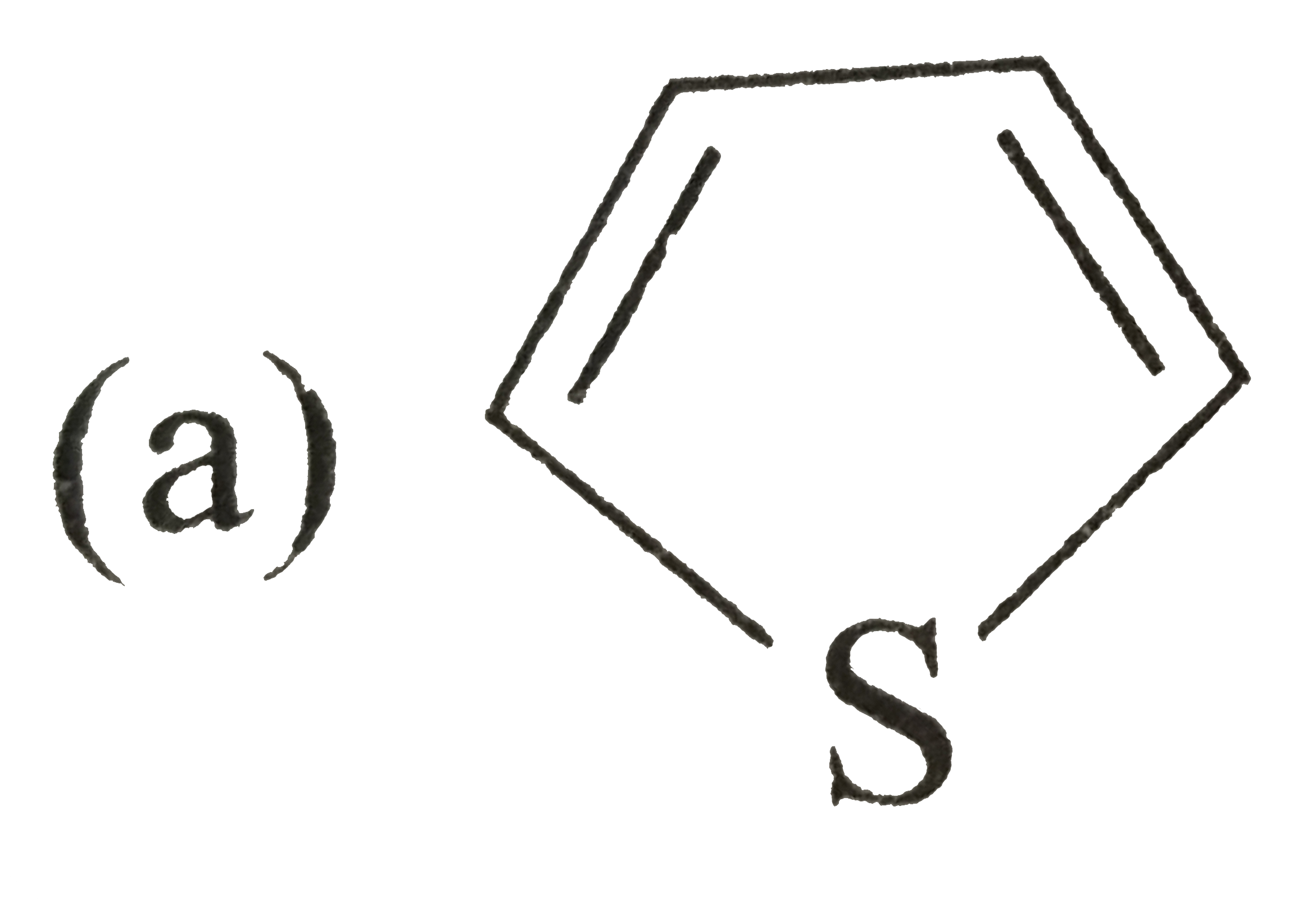
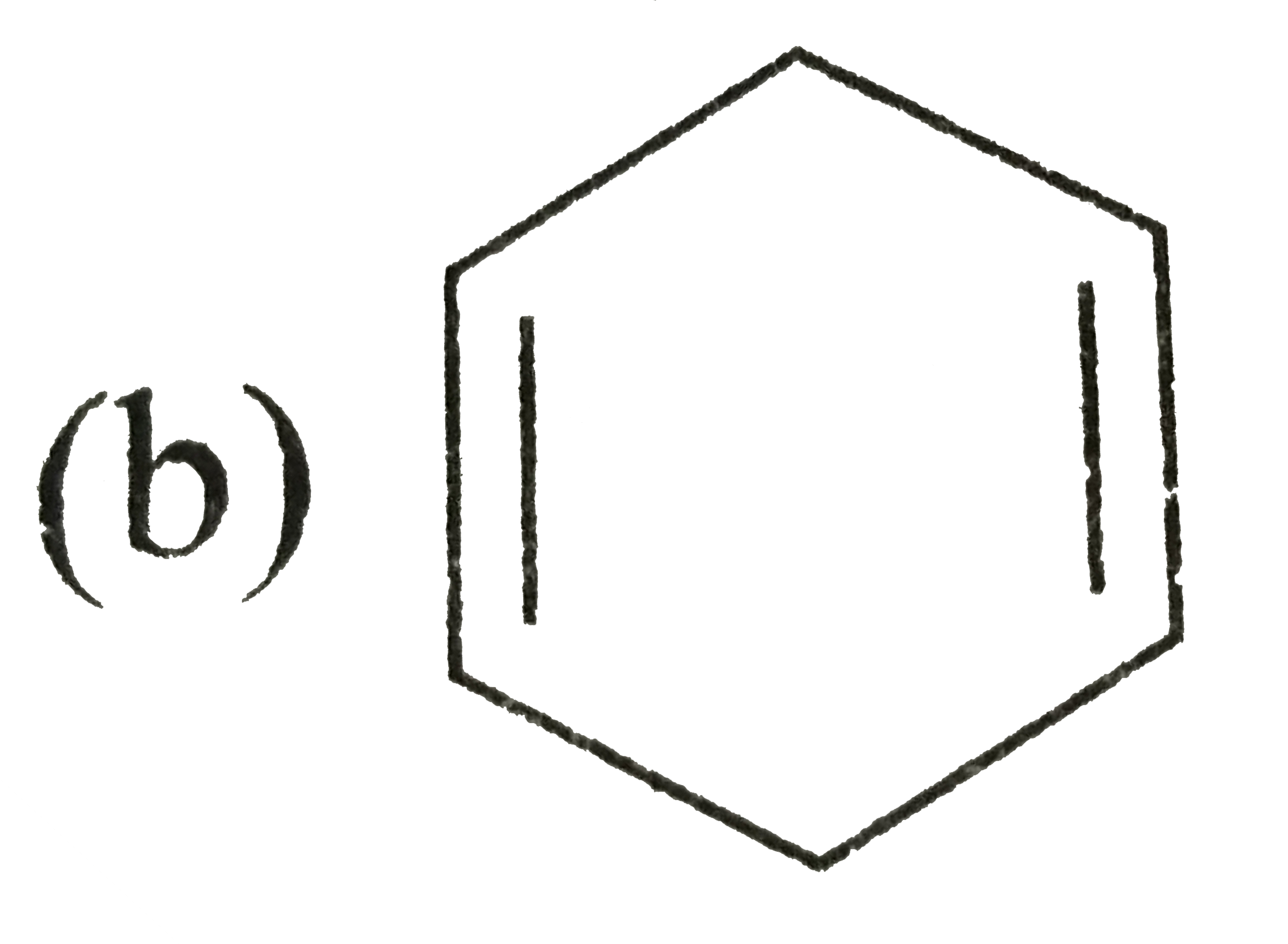
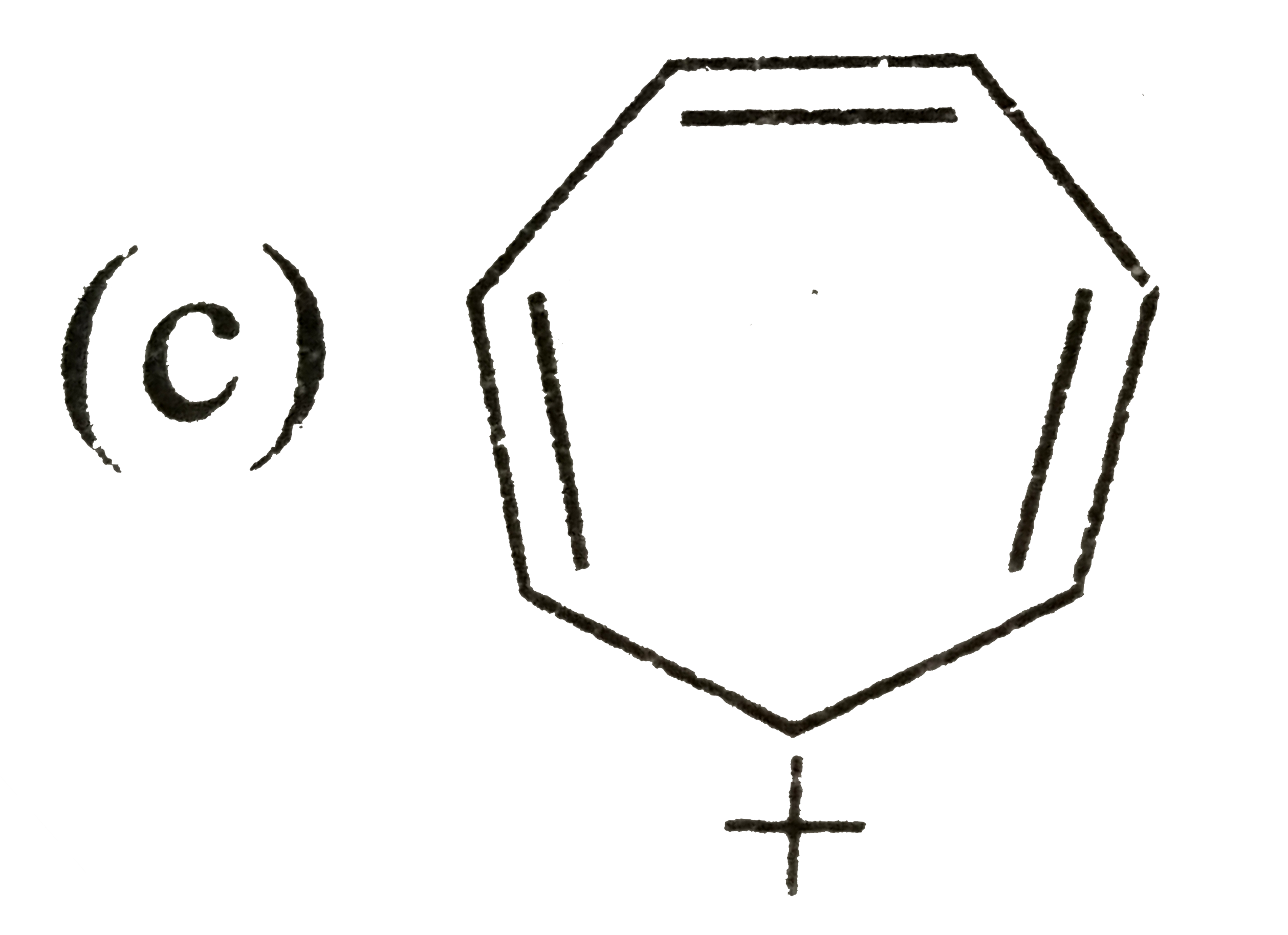
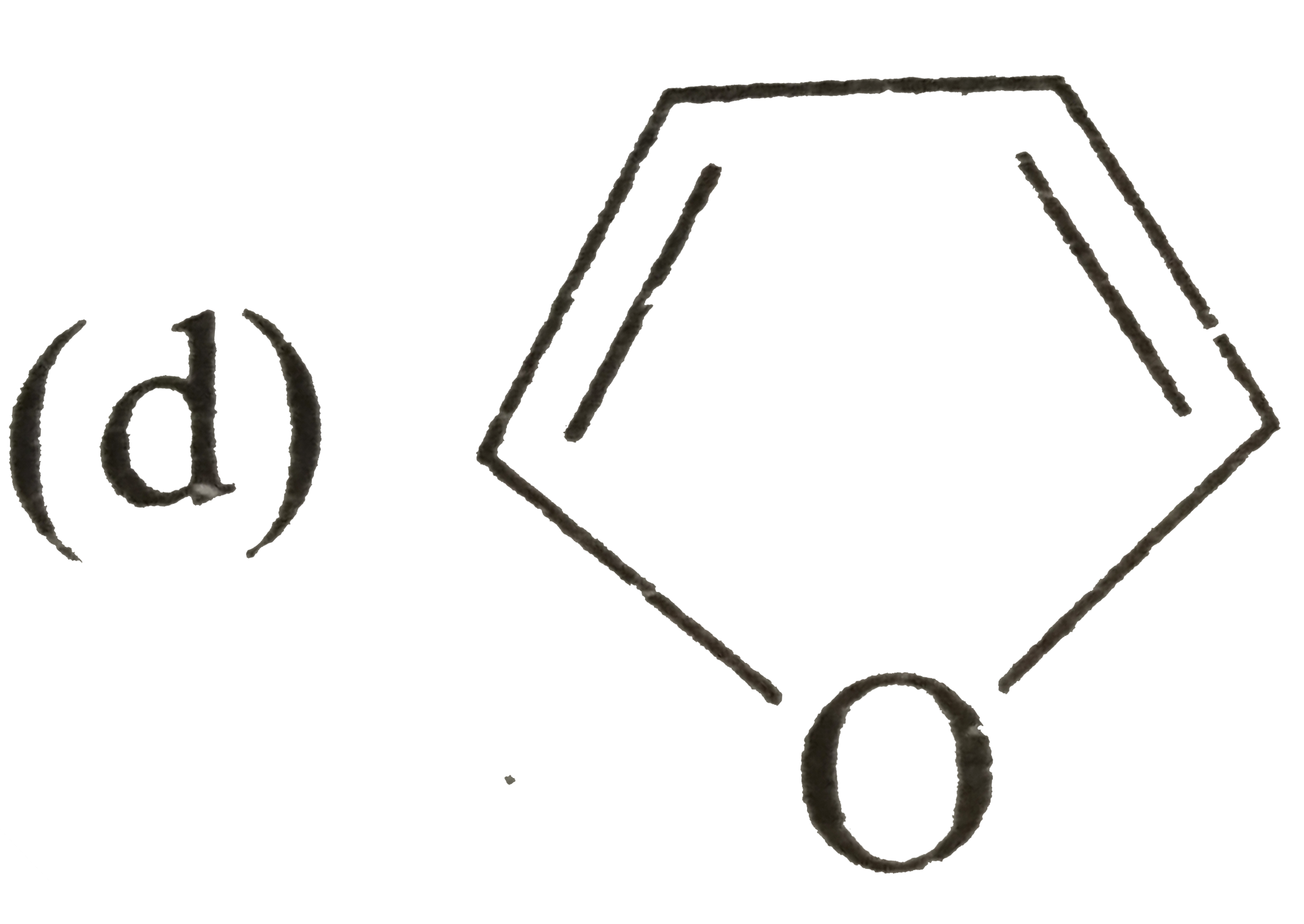
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Level 2 (Q.1 To Q.25)|25 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Level 2 (Q.26 To Q.50)|25 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Subjective Type Problems|9 VideosAMINES
HIMANSHU PANDEY|Exercise Subjective Type Problems|5 VideosBIOMOLECULES
HIMANSHU PANDEY|Exercise Match The Column|6 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-AROMATIC HYDROCARBONS-Level 1 (Q.26 To Q.50)
- The best method for the perparation of chlorobenzene is :
Text Solution
|
- Which of the following reaction is called 'Schotten- Baumann' reactio...
Text Solution
|
- Which of the following is not correctly matched ?
Text Solution
|
- Indenify the end product (b) of the following sequence of reaction
Text Solution
|
- Benzene can be obatined by :
Text Solution
|
- Point out incorrect statement about resonace.
Text Solution
|
- The major product X is :
Text Solution
|
- overset(Na^(o+)NH(2))toA, major product A and reaction R are :
Text Solution
|
- In the sulphonation, acetylation and formlylation of benzene the gro...
Text Solution
|
- Benzoic acid may be prepared by the oxidation of :
Text Solution
|
- Chloral product.The product is :
Text Solution
|
- Which of the following is not an aromatic compound ?
Text Solution
|
- The correct order of stability of ions is :
Text Solution
|
- Number of pi electrons present in naphthalene is :
Text Solution
|
- Dipole moment of which compound will zero ?
Text Solution
|
- Xoverset(Cl(2))to Benzotrichloride overset("Hydrolysis")toY. What are...
Text Solution
|
- Which of the following reaction is not an example of electrophilic s...
Text Solution
|
- Major product of this reaction will be :
Text Solution
|
- Which of the following may best be called aromatic ?
Text Solution
|
- Amongst the ions, the aromatic character is shown by :
Text Solution
|