Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-HYDROGEN-Three marks questions and answers
- Explain occurrence of hydrogen.
Text Solution
|
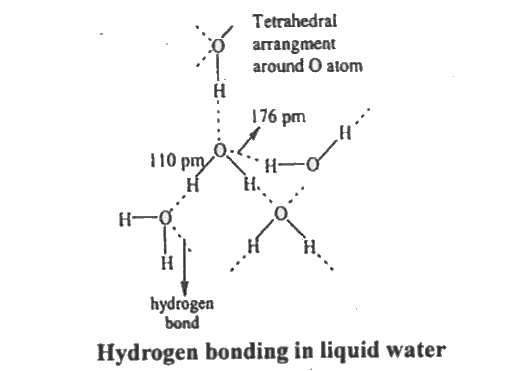
- Explain the structure of water molecule ?
Text Solution
|
- In what respects does hydrogen resemble alkali metals ? How does it re...
Text Solution
|
- In what respects H(2) differs from alkali metals and halogens ?
Text Solution
|
- Dihydrogen is considered as fuel Explain.
Text Solution
|
- Explain hydrogen economy.
Text Solution
|
- What different methods are used for softening the hard water ? Explain...
Text Solution
|
- Give four uses of hydrogen peroxide.
Text Solution
|
- How is hydrogen peroxide prepared industrially ? Explain why it is sto...
Text Solution
|
- Distinguish between temporary hardness permanent hardness.
Text Solution
|
- What happens when lead sulphide is reacted with hydrogen peroxide solu...
Text Solution
|
- Why hard water does not form lather with soap ? What advantage has soa...
Text Solution
|
- Advantage of soap over detergent : Soap are biodegradable whereas dete...
Text Solution
|
- The mixture of hydrazine and hydrogen peroxide with copper (II) cataly...
Text Solution
|
- Hydrogen peroxide acts both as an oxidizing agent as a reducing agent ...
Text Solution
|