Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-CHEMICAL KINETICS -PROBLEMS SECTION
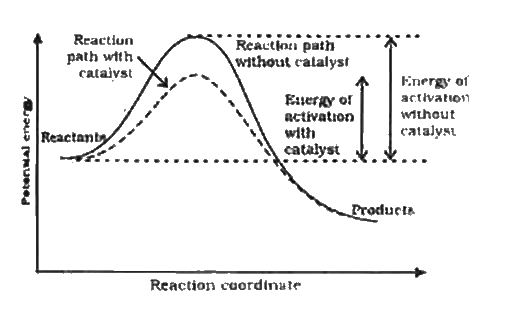
- How catalyst increases rate of a reaction.
Text Solution
|
- For the reaction R to P, the concentration of a reactant changes from ...
Text Solution
|
- In a reaction 2A product, the concentration of A decreases from 0.5 mo...
Text Solution
|
- The conversion of molecules X to Y follows second order kinetics. IF c...
Text Solution
|
- The initial concentration of N(2)O(5) in the following first order rea...
Text Solution
|
- The rate constant of a certain first order reaction is 200S^(-1). What...
Text Solution
|
- A certain first order reaction is half completed in 46 min. Calculate ...
Text Solution
|
- Show that in case of a first order reaction, the time taken for comple...
Text Solution
|
- Show that t(99%) = 2 xx t(90%)
Text Solution
|
- Rate constant of a first order reaction is 0.0693 min^(-1). Calculate ...
Text Solution
|
- The rate constant for a first order reaction is 60s^(-1). How much tim...
Text Solution
|
- A first order reaction takes 40 min for 30% decompositon.
Text Solution
|
- The half life for radioactive decay of ""^(14)C is 5730 years. An arch...
Text Solution
|
- A first order reaction is found to have a rate constant K=5.5xx10^(-14...
Text Solution
|
- A first order reaction has a rate constant 1.15 xx 10^(-3)s^(-1). How ...
Text Solution
|
- Time required to decompose SO(2)Cl(2) to half of its initial amount is...
Text Solution
|
- The rate constant of a particular reaction doubles when the temperatur...
Text Solution
|
- The rate constant of a raction at 500 K and 700 K are 0.02 s^(-1) and ...
Text Solution
|
- The rate of a chemical reaction doubles for an increase of 10K in abso...
Text Solution
|
- The rate of a reaction becomes four times when the temperature changes...
Text Solution
|