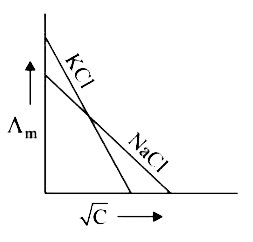
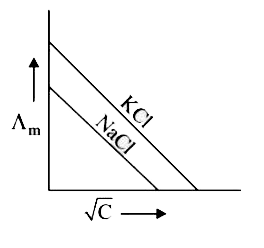
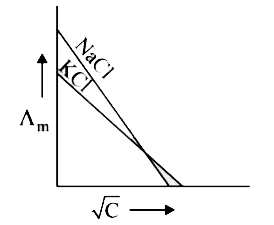
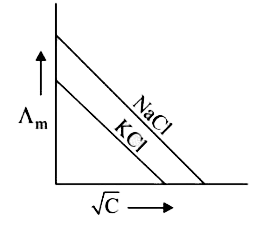
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which one of the following graphs between molar conductivity (A(m)) ...
Text Solution
|
- A graph was plotted between molar conductivity of various electrolytes...
Text Solution
|
- For electrolyte A(x)B(y) which is /are not correct relation between mo...
Text Solution
|
- Which of the following has highest molar conductivity
Text Solution
|
- Which one of the following graphs between molar conductivity (A(m)) ...
Text Solution
|
- Draw a graph between Lambda(m)^(@) and sqrtC for strong and weak elect...
Text Solution
|
- Above plot represents the variation of molar conductance against sqrtC...
Text Solution
|
- Which one of the following has the highest molar conductivity?
Text Solution
|
- मोलर चालकता (Lambdam) तथा sqrtC के बीच बने ग्राफों में से कौन-सा सही ह...
Text Solution
|