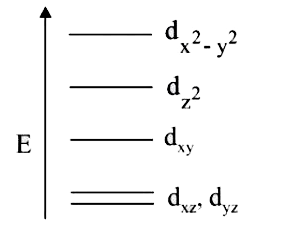
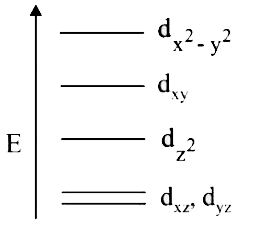
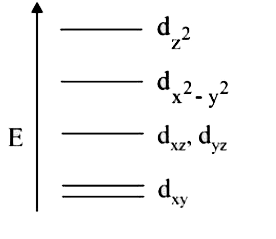
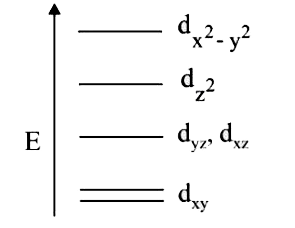
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Complete removal of both the axial ligands (along the z-axis) from an ...
Text Solution
|
- From an octahedral splitting arrangement if two ligans present along Z...
Text Solution
|
- When the valence d-orbitals of the central metal ion split in energy i...
Text Solution
|
- In the formation of octahedral complex , ligands approach towards and...
Text Solution
|
- According to CFT ,five d-orbitals of an octahedral complex split to gi...
Text Solution
|
- Complete removal of both the axial ligands (along the z-axis) from an ...
Text Solution
|
- Which of the following statements is/are correct? (i) In octahedral co...
Text Solution
|
- In the formation of octahedral complex, ligands approach towards and o...
Text Solution
|
- अष्टफलकीय संकर से (z-अक्ष के साथ) दोनों अक्षीय लिगैण्ड के पूर्ण रूप से...
Text Solution
|