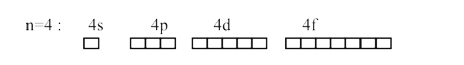A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The number of orbitals associated with quantum number n = 4,ms=+1/2 is...
Text Solution
|
- In an atom, the total number of electrons having quantum numbers n =...
Text Solution
|
- what is the total number of orbitals associated with the principal qua...
Text Solution
|
- In an atom, the total number of electrons having quantum numbers n =...
Text Solution
|
- एक परमाणु में , क्वांटम संख्या n=4,[m(l)]=1 तथा m(s) = - (1)/(2) र...
Text Solution
|
- The number of orbitals associated with quantum numbers n =4 and m (8) ...
Text Solution
|
- The number of orbitals associated with quantum number n = 4, ms=+1/2 i...
Text Solution
|
- What is the total number of orbitals associated with the principal qua...
Text Solution
|
- एक परमाणु में क्वाण्टम संख्या n=4,|m(l)|=1 तथा m(s)=-1//2 रखने वाले इल...
Text Solution
|