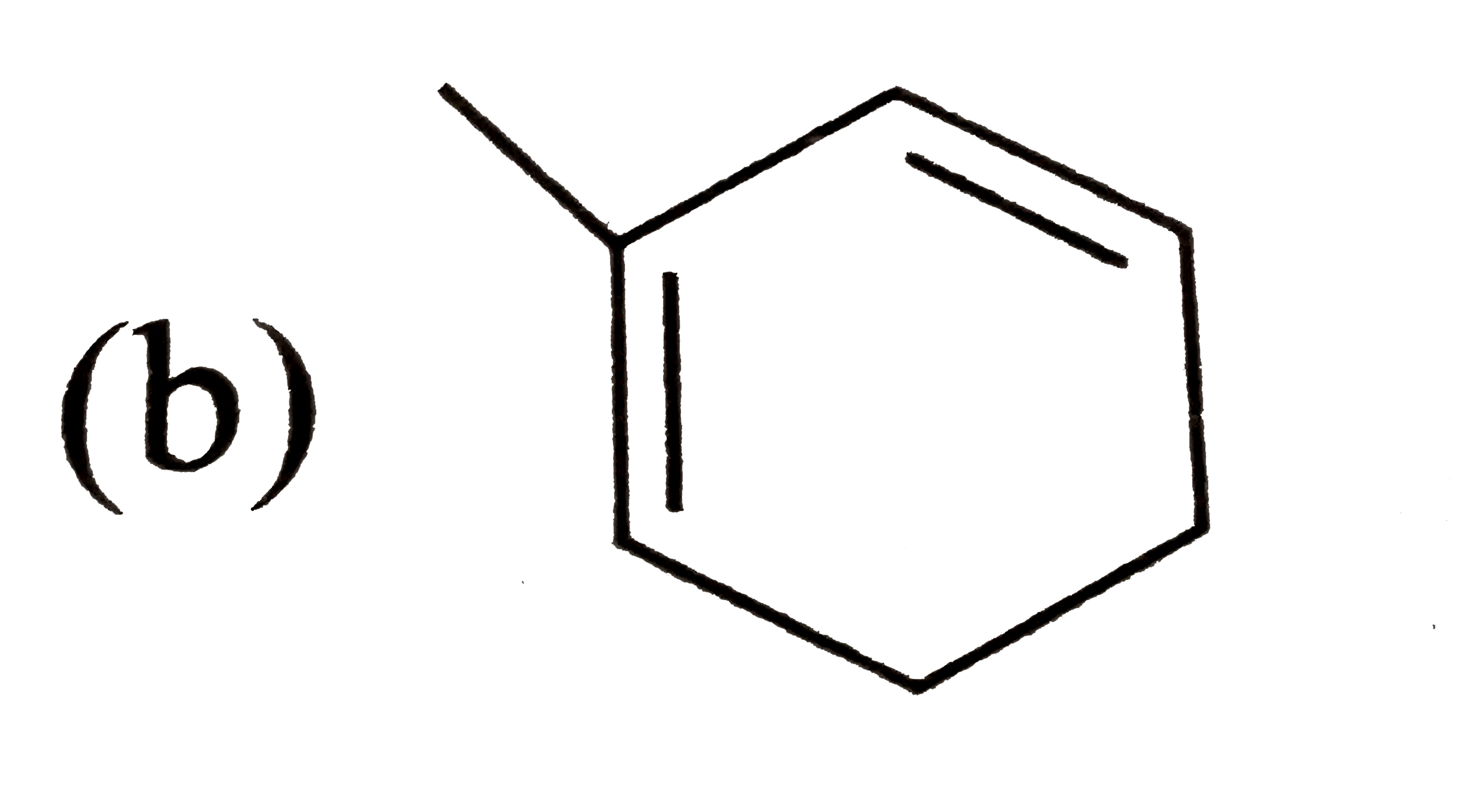
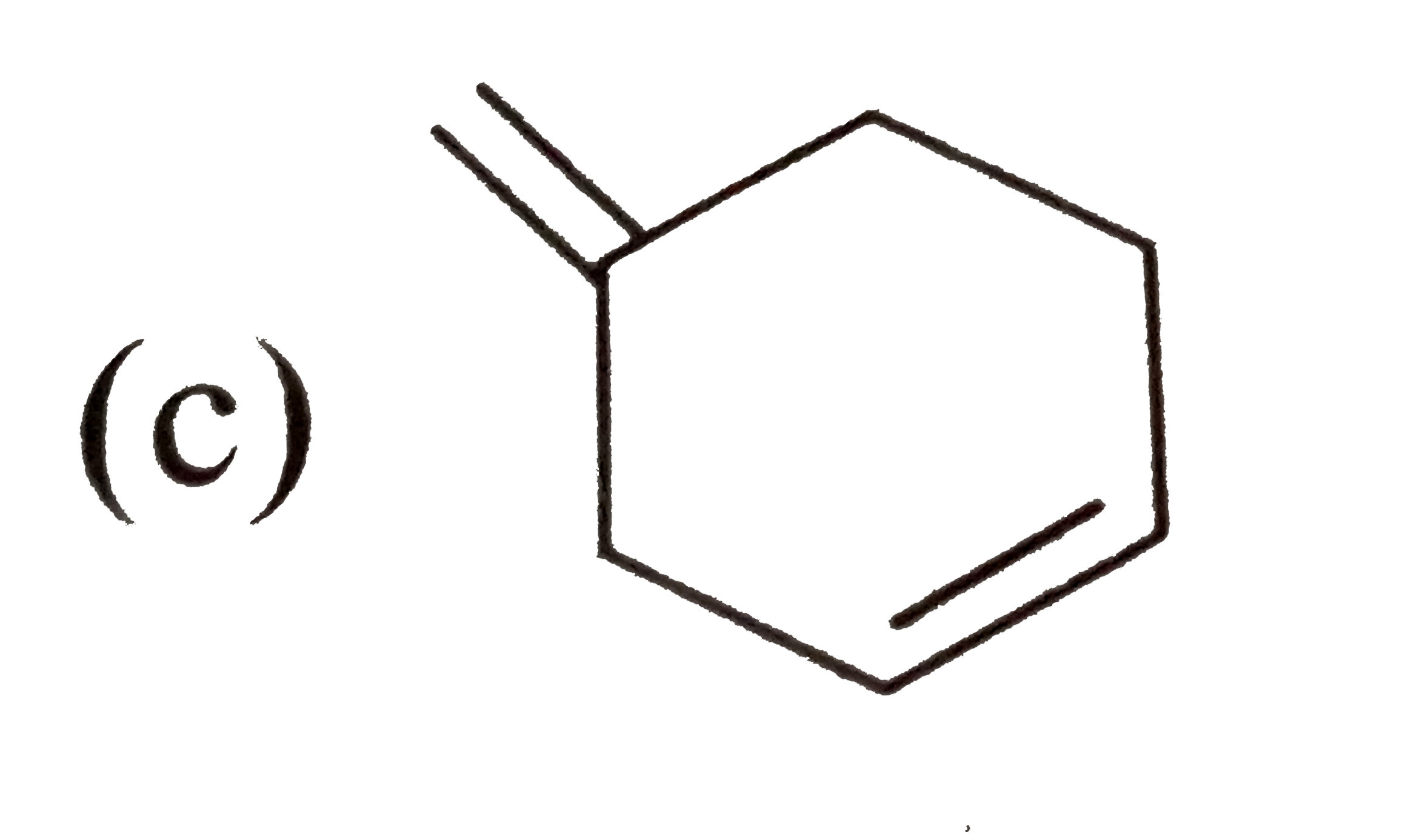
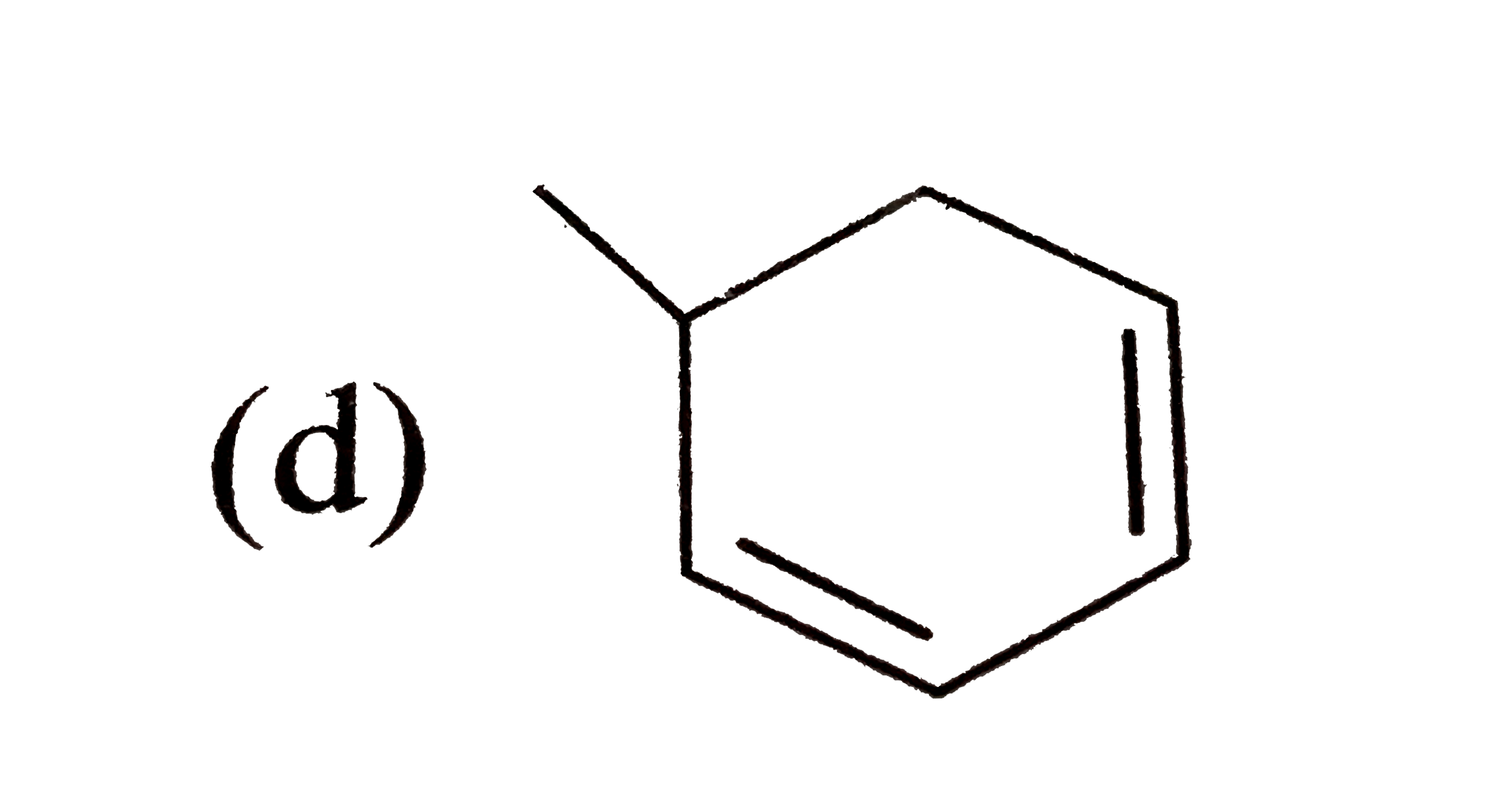
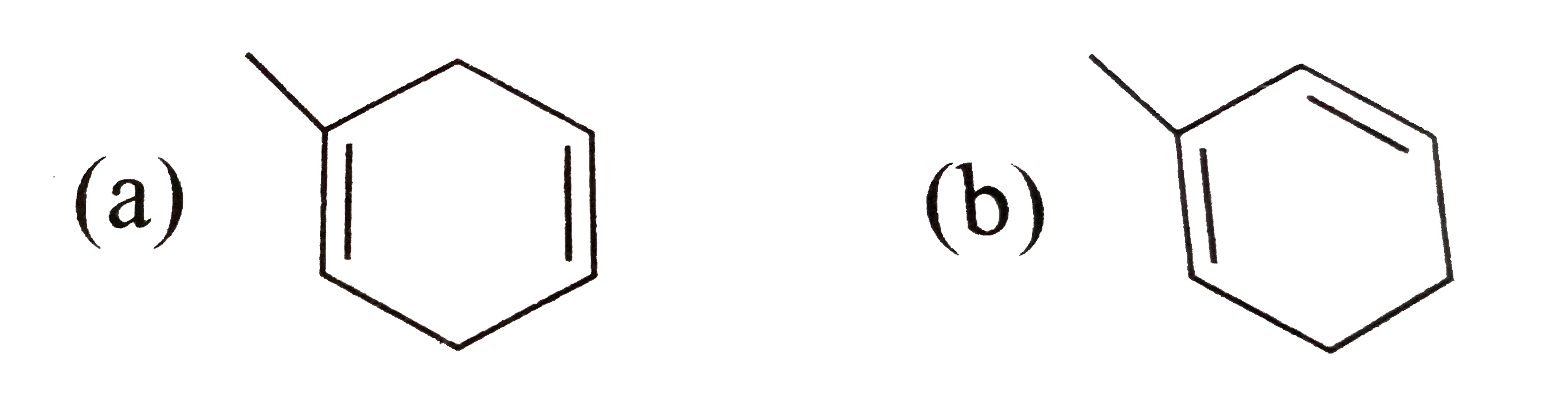A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.201 To Q.205)|5 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise More Than One Correct (Q.1 To Q.25)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.151 To Q.175)|25 VideosCHEMISTRY IN DAILY LIFE
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|7 VideosHALIDES
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-GENERAL ORGANIC CHEMISTRY-Level 2 (Q.176 To Q.200)
- Which of the following is the correct order for bond energy for C-H bo...
Text Solution
|
- Which of the following orders is correct for the magnitude of +M power...
Text Solution
|
- Which of the following order is correct for heat of hydrogenation of t...
Text Solution
|
- Which of the following orders is correct for the stability of these ca...
Text Solution
|
- Which of the following anions is resonance destabilized?
Text Solution
|
- Complete the following reaction
Text Solution
|
- Hyperconjugation occurs in :
Text Solution
|
- The most stable resonating structure of the following molecule is :
Text Solution
|
- The decreasing order of nucleophilies among the nucleophie :
Text Solution
|
- Which of the following compounds have a diople moment?
Text Solution
|
- Which of the following is the least stable?
Text Solution
|
- Among the following which is more reactive toward AgNO(3)?
Text Solution
|
- Identify the compound which cantian most acidic hydrogen :
Text Solution
|
- Identify the most stable structuer among the following :
Text Solution
|
- The correct stability order of the give canonical structure is :
Text Solution
|
- Arrange the following in increasing order of basicity :
Text Solution
|
- Compare acidic strength of the following compound.
Text Solution
|
- Select the most stable structure among following :
Text Solution
|
- Identify the compound which is not aromatic :
Text Solution
|
- Find out anti aromatic compound among the following :
Text Solution
|