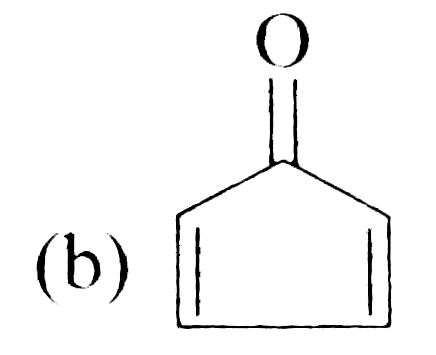
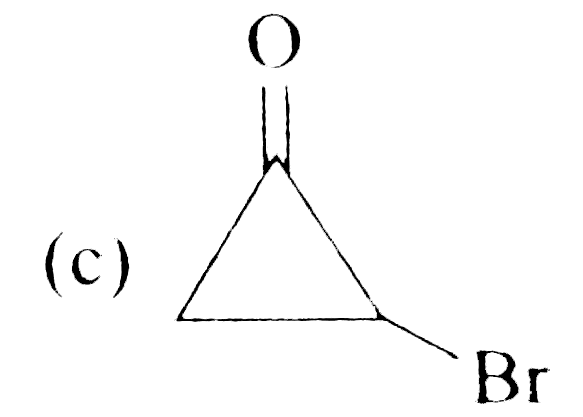
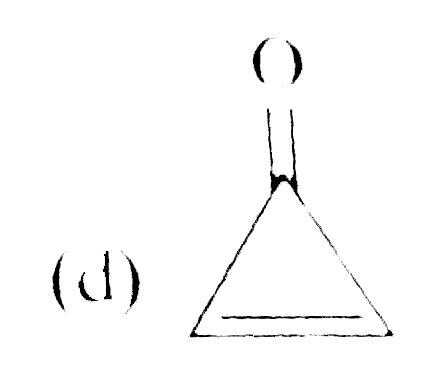
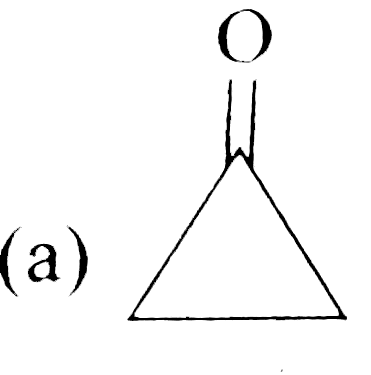A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBONYL COMPOUNDS
HIMANSHU PANDEY|Exercise Level 2 (Q.26 To Q.50)|25 VideosCARBONYL COMPOUNDS
HIMANSHU PANDEY|Exercise Level 2 (Q.51 To Q.75)|25 VideosCARBONYL COMPOUNDS
HIMANSHU PANDEY|Exercise Level 1 (Q.51 To Q.1)|2 VideosCABOXYLIC ACIDS AND ITS DERIVATIVES
HIMANSHU PANDEY|Exercise Subjective Type Problems|8 VideosCHEMISTRY IN DAILY LIFE
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|7 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-CARBONYL COMPOUNDS-Level 2 (Q.1 To Q.25)
- In the given reaction X is :
Text Solution
|
- Secondary amine react with carbonyl compound to give :
Text Solution
|
- In the given reaction A and B will respectively be :
Text Solution
|
- Arrange the compounds in order of decreasing reactivity for nucleophil...
Text Solution
|
- In the given reaction Identify P and Q :
Text Solution
|
- Which carbonyl group of the given compound is most reactive for nucleo...
Text Solution
|
- Which carbonyl compound has maximum dipole moment ?
Text Solution
|
- In the given reaction the main product will be :
Text Solution
|
- In the given reaction the main product will be :
Text Solution
|
- Arrange the stability of given gemdiols is decreasing order :
Text Solution
|
- Which one of the following compounds would form most stable hydrate ?
Text Solution
|
- Which of the following structures contains a hemiacetal group ?
Text Solution
|
- Which of the following compounds would give positive Fehling's solutio...
Text Solution
|
- Which of the following cabonyl compounds when treated with dilute acid...
Text Solution
|
- In the given reaction P will be :
Text Solution
|
- (X) will be:
Text Solution
|
- In the given reaction (X) will be :
Text Solution
|
- In the given reaction (X) will be :
Text Solution
|
- What are A, B and C in the given reaction ?
Text Solution
|
- identify Q :
Text Solution
|