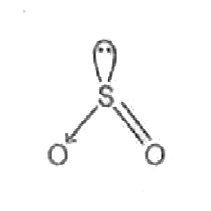
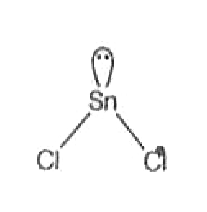A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-TS EAMCET 2019 (6 MAY SHIFT 1)-CHEMISTRY
- Assertion (A) While going from left to right of the periodic table, th...
Text Solution
|
- Which one among the following statements is/are not correct? I. Ba c...
Text Solution
|
- Which of the following have linear structure I. SnCl(2) II. BeF(2) ...
Text Solution
|
- Match the following : The correct answer is
Text Solution
|
- Which of the following is/are correct for Boyle's law ?
Text Solution
|
- The compressibility factor (Z) of a gas at critical state is (T(e)=(...
Text Solution
|
- The number of moles of H(2) which is required to produce 10 moles of N...
Text Solution
|
- Match the following : The correct answer is
Text Solution
|
- What is the standard enthalpy of rection (in kJ) when two moles of Fe(...
Text Solution
|
- In which of the following plots, an endothermic reaction if correctly ...
Text Solution
|
- What is the order of relative basic strength of ClO(2)^(-),ClO(3)^(-),...
Text Solution
|
- How many water molecules present in CuSO(4).5H(2)O are hydrogen bonded...
Text Solution
|
- Which gas/gases evolve (s) when sodium metal is reacted with at room t...
Text Solution
|
- A and B are formed when borax, is dissolved in water. C and B are form...
Text Solution
|
- How many nearest neighbours are there for Si and O atoms in quartz cry...
Text Solution
|
- Match the following : The correct answer is
Text Solution
|
- Which of the following method is adopted to obtain gasoline from crude...
Text Solution
|
- Which of the following statements is correct in the Kolbe's electrolys...
Text Solution
|
- The correct order of rates of C-Br bond ionisation of the following br...
Text Solution
|
- If an element having atomic number 96 crystallises in cubic lattice wi...
Text Solution
|