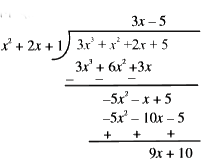Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY : BASIC PRINCIPLES AND TECHNIQUES
CHHAYA PUBLICATION|Exercise SHORT - TYPE QUESTIONS|17 VideosMODEL QUESTIONS PAPER
CHHAYA PUBLICATION|Exercise SET III (SECTION II) (GROUP D )|12 VideosP-BLOCK ELEMENTS
CHHAYA PUBLICATION|Exercise PRACTICE SET 7 (ANSWER THE FOLLOWING QUESTIONS) : -|17 Videos
Similar Questions
Explore conceptually related problems
CHHAYA PUBLICATION-ORGANIC CHEMISTRY : BASIC PRINCIPLES AND TECHNIQUES -WARM UP EXERICSE
- Which of the following compounds can be reperesented as a resonance hy...
Text Solution
|
- Why are the three carbon - oxygen bonds in carbonate (CO""^(2^(-))/(3)...
Text Solution
|
- Which one between phenol and cyclohexanol is more acidic and why?
Text Solution
|
- Arrange the following ions in order of increasing stability and give y...
Text Solution
|
- Which one between 2 - methylbut - 2 - ene and 2 - methylbut - 1 - ene ...
Text Solution
|
- The C - C bond in acetaldehyde (CH(3)CHO)) is shorter than that in eth...
Text Solution
|
- Arrange the following isomeric alkenes in order of increasing stabili...
Text Solution
|
- Which one of the following two conformations of butane is more stable ...
Text Solution
|
- Which of the 2 geometric isomers of Me(3)C CH=CHCMe(3) has higher hea...
Text Solution
|
- Explain the following observation
Text Solution
|
- Label the following carbocations as 1^(@), 2^(@) or 3^(@) :
Text Solution
|
- Arrange the following carbocations in order of increasing stability an...
Text Solution
|
- Which of the carbocations is the most stable? (i) CH(3)CH(2)overset(...
Text Solution
|
- Which one of the two carbanions overset(Theta)(triangle),overset(Theta...
Text Solution
|
- Which one between the two CH(3)COoverset(Theta)(C)H(2) and CH(3)COove...
Text Solution
|
- Arrange the following free radicals in order of increasing stability ...
Text Solution
|
- What are the shapes of the free radicals overset(*)(C)H(3),overset(*)...
Text Solution
|
- What is homolytic bond fission?
Text Solution
|
- Which one between C(6)H(5)CH(3)andCH(4) has lower C(sp^(3))-H bond dis...
Text Solution
|
- Arrange the following carbocation in order of increasing stability an...
Text Solution
|