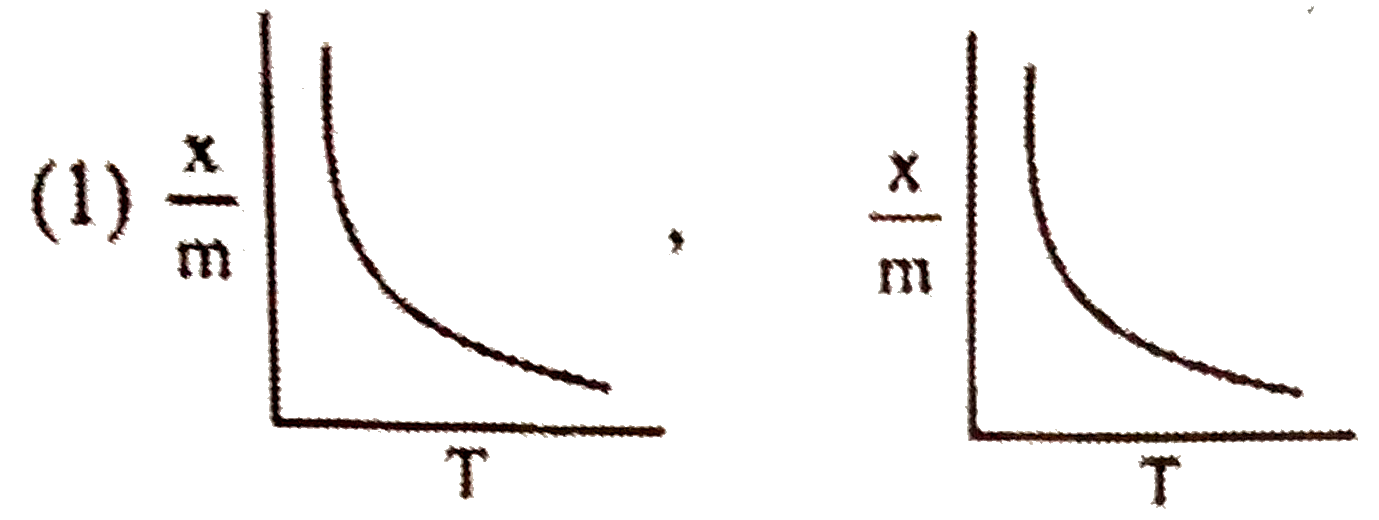
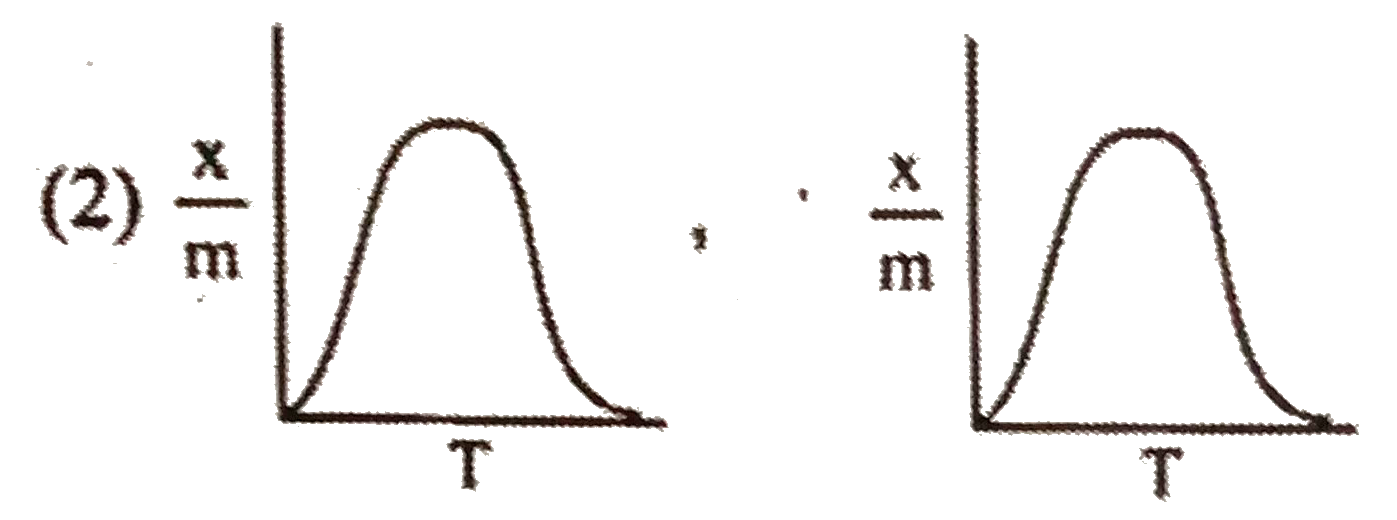
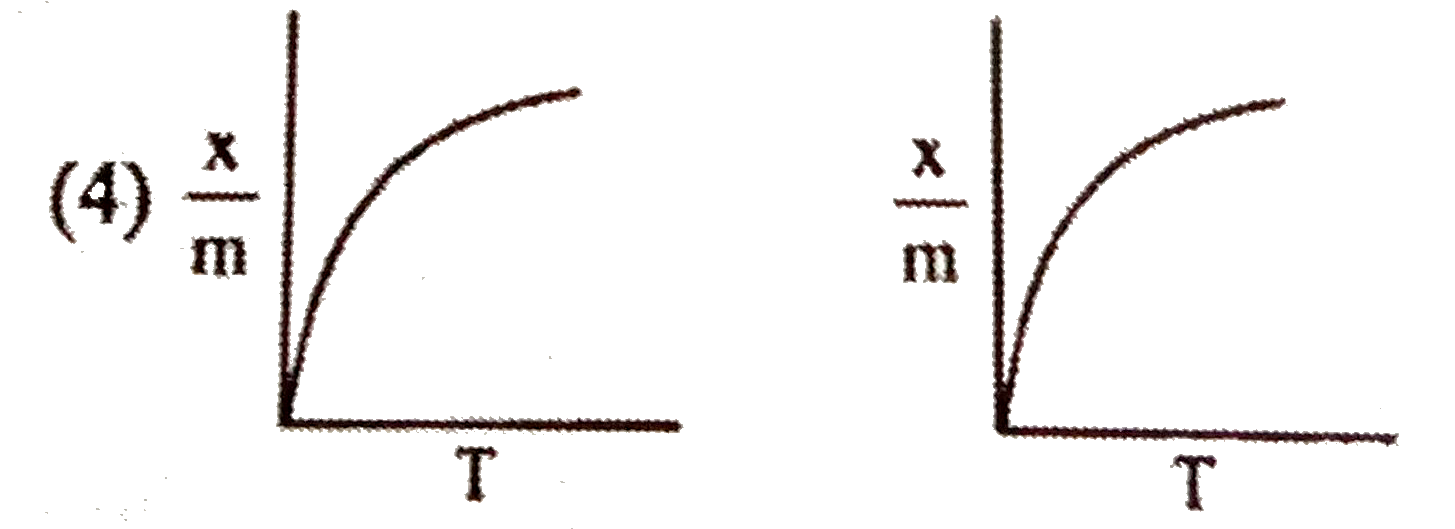
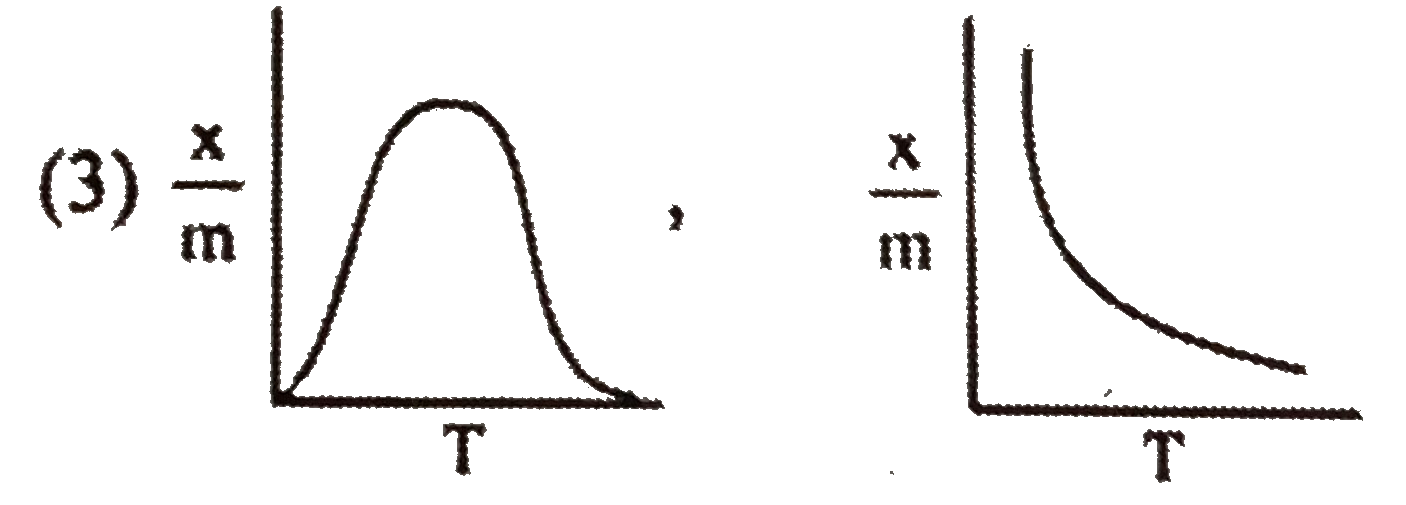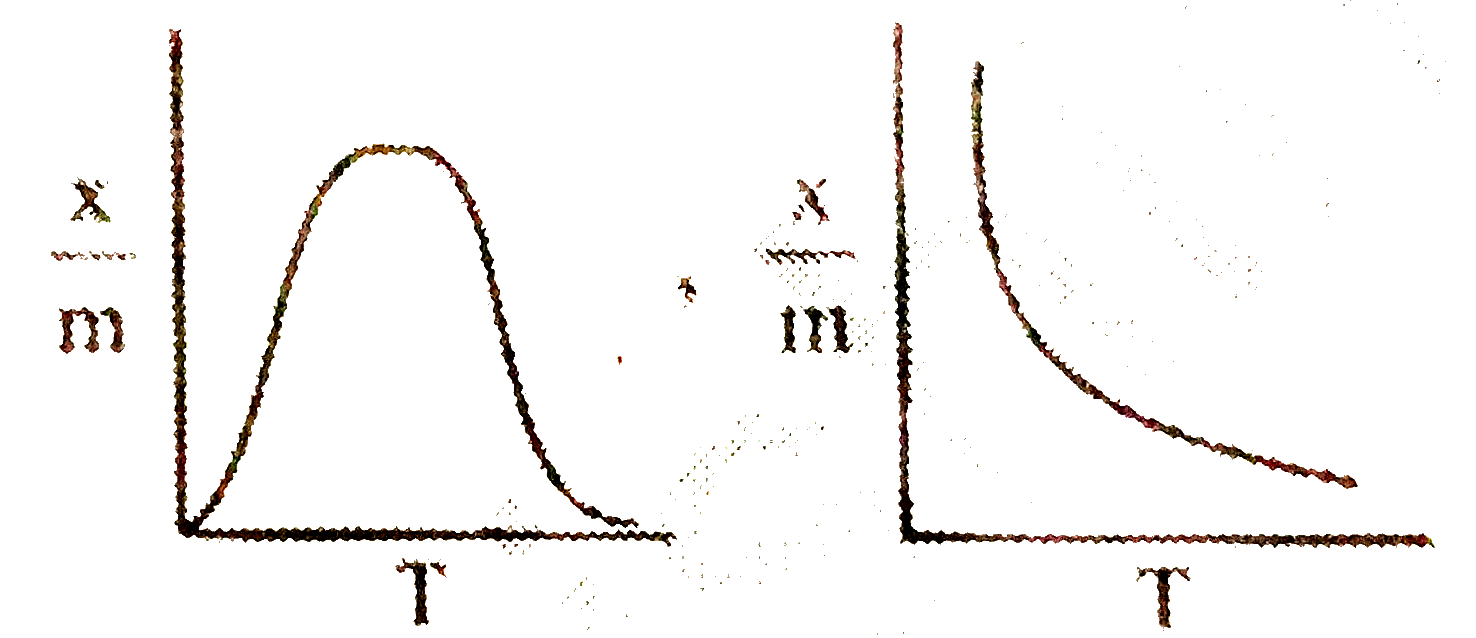A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CAREER POINT-MOCK TEST 6-Part (B) (CHEMISTRY)
- {:(MnO(2)+KOH+O(2)rarrunderset(("green"))(A)+H(2)O),(A+Cl(2)rarrunders...
Text Solution
|
- Incorrect order of boiling point is -
Text Solution
|
- The electron affinity of the following element can be arranged -
Text Solution
|
- (A) would be -
Text Solution
|
- Which of the following statement are incorrect about phenol-formaldehy...
Text Solution
|
- Product formed in above reaction is -
Text Solution
|
- Which of the following statement is not true for detergants ?
Text Solution
|
- The incorrect structure of glucine at given pH are-
Text Solution
|
- Which of the following sets of quantum numberrs discribes the elecron ...
Text Solution
|
- An aqueous solution of ethanol has density 1.025 g/ mL and it is 2 M. ...
Text Solution
|
- Select correct adsorption isobars for chemisorption and physisorption ...
Text Solution
|
- Which of the following is involved in the extraction of Ag from argent...
Text Solution
|
Text Solution
|
- In which of the following 2^(nd) anion is more stable than first ?
Text Solution
|
- Find out the product of following reaction : X and Y are :
Text Solution
|
- CH(3)-underset(OH)underset(|)overset(CH(3))overset(|)(C)-underset(NH(2...
Text Solution
|
- How many molecules of RMgX are consumed in the above given reaction ?
Text Solution
|
- In a hydrogen atom, the transition takes place from n = 3 to n = 2 ....
Text Solution
|
- If 30g of a solute of molecular weight 154 is dissolved in 250 g of be...
Text Solution
|
- If 50% of CO(2) converts to CO at the following equilibrium : 1/2C(...
Text Solution
|