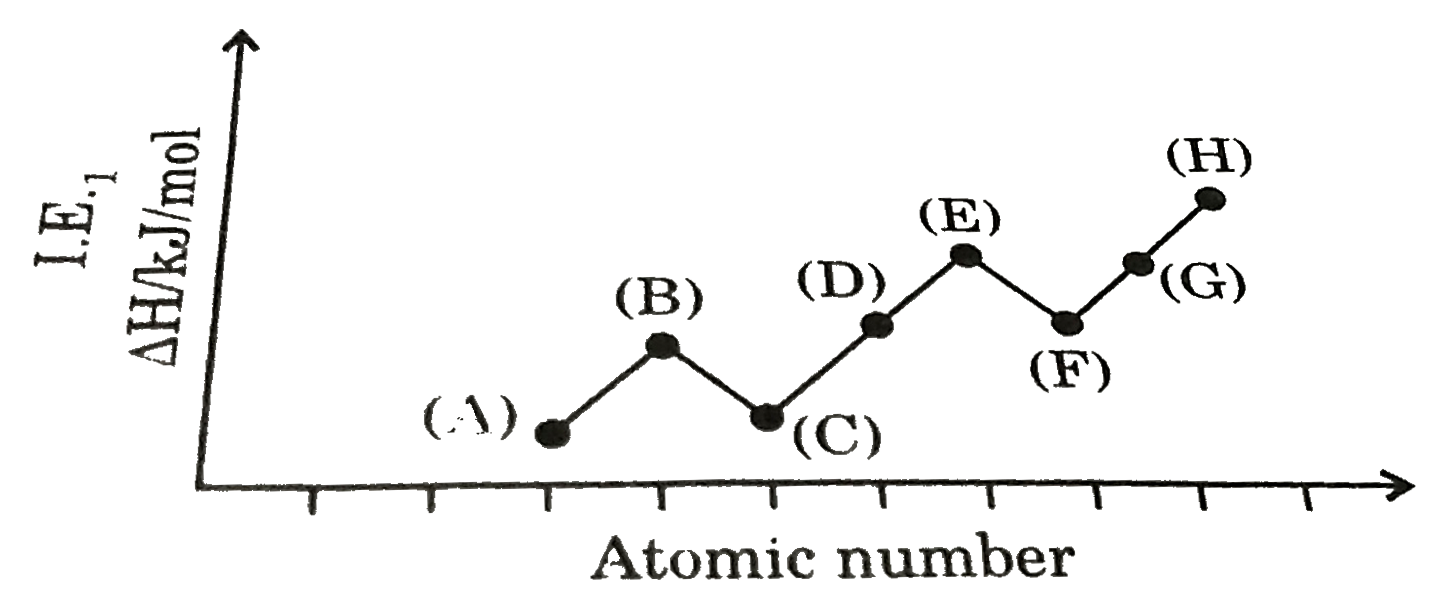
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC TABLE
GRB PUBLICATION|Exercise Comprehension 14|1 VideosPERIODIC TABLE
GRB PUBLICATION|Exercise Comprehension 15|1 VideosPERIODIC TABLE
GRB PUBLICATION|Exercise Comprehension 12|1 VideosNOMENCLATURE AND CLASSIFICATION
GRB PUBLICATION|Exercise Subjective Type|24 VideosPRACTICAL ORGANIC CHEMISTRY
GRB PUBLICATION|Exercise Exercise 4 (Matrix match type )|1 Videos
Similar Questions
Explore conceptually related problems