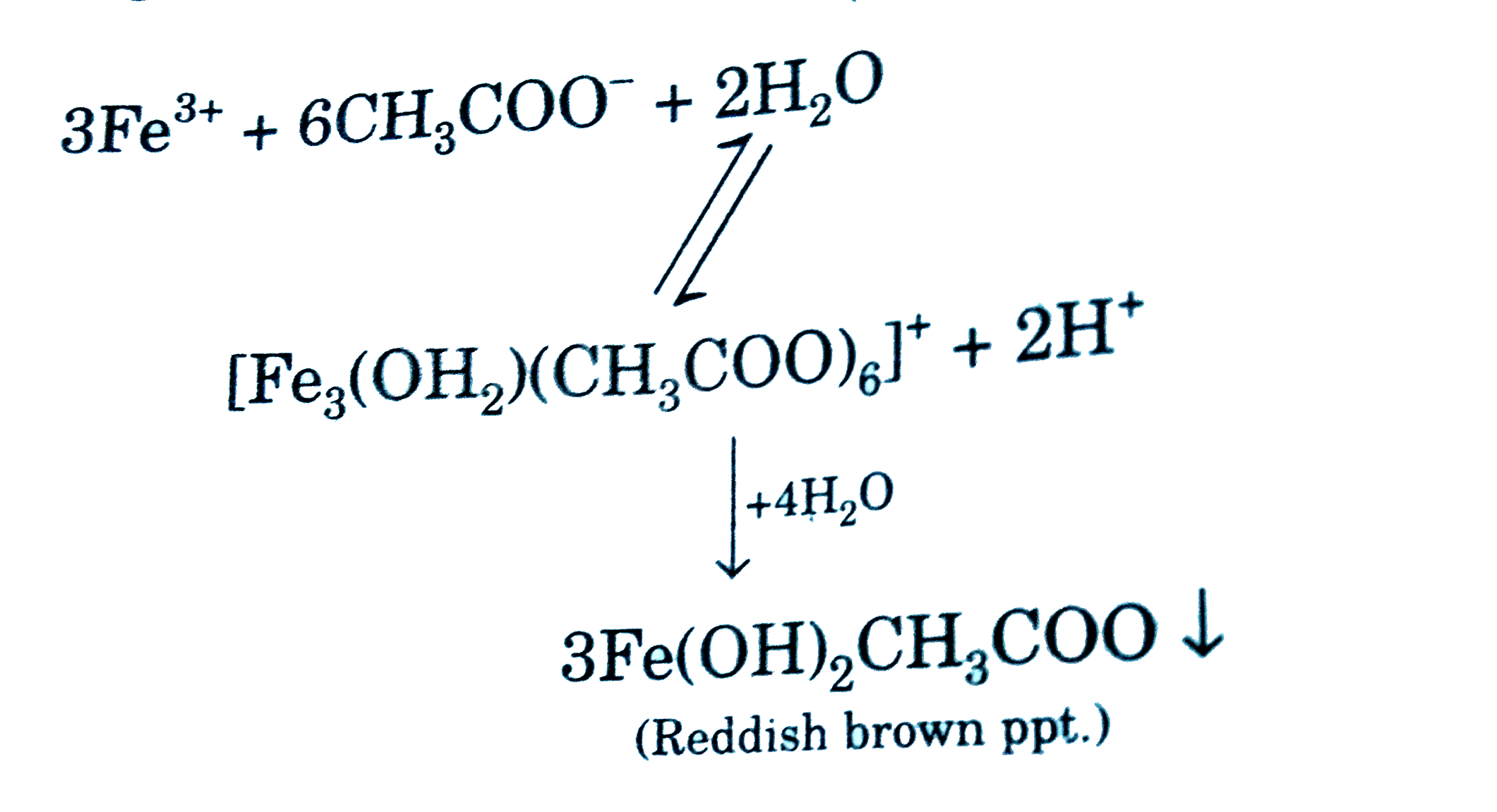A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SALT ANALYSIS
GRB PUBLICATION|Exercise Multiple Objective Type|129 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise Comprehension 1|1 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise L: Miscellaneous|40 VideosS BLOCK ELEMENTS
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|9 VideosTHERMODYNAMICS
GRB PUBLICATION|Exercise All Questions|704 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SALT ANALYSIS-Reasoning Type
- Statement-1: White precipitates of AgCI and PbCI(2) can be separated b...
Text Solution
|
- Statement-1 :Cu^(2+) and Cd^(2+) ions form complexes with excess of p...
Text Solution
|
- Statement-1: Aqueous solution containing sodium acetate and ferric chl...
Text Solution
|
- Statement-1: Presence of Fe^(3+) in an aqueous solution can be confirm...
Text Solution
|
- Statement-1: Chromium ions give gree precipitate of Cr(OH)(3) with Na(...
Text Solution
|
- Statement-1: In neutral solution, there is partial precipitation of zi...
Text Solution
|
- Statement-1: Zinc ions are not precipitated by ammonia solution as zin...
Text Solution
|
- Statement-1: Borax bead test can be used for the identification of col...
Text Solution
|
- Statement-1: Potassium chromate solution in acetic acid precipitates o...
Text Solution
|
- Statement-1: Solution of Ca^(2+) ions does not give any precipitate wi...
Text Solution
|
- Statement-1: Ca^(2+) ions do not form any precipitate with ammonia sol...
Text Solution
|
- Statement-1: When HCO(3)^(-) and CO(3)^(2-) ions are present together,...
Text Solution
|
- Statement-1: NaHCO(3) is the least soluble alkali bicarbonate. State...
Text Solution
|
- Statement-1: LiHCO(3) cannot exist in solid form. Statement-2: Li(2)...
Text Solution
|
- Statement-1: CO(2)+K(2)Cr(2)O(7)rarr on reaction. Statement-2: Carbo...
Text Solution
|
- Statement-1: Initially there is no ppt.when AgNO(3) is added to Na(2)S...
Text Solution
|
- Statement-1: In the brown ring compound, Fe is in the +1 oxidation sta...
Text Solution
|
- Statement-1: Brown ring test can be done for NO(3)^(-) in presence of ...
Text Solution
|
- Statemen-1: NH(4)^(+) and K^(+) cations can be distinguished by using ...
Text Solution
|
- Statement-1: NO(3)^(-) and NO(2)^(-) both do not give brown fumes with...
Text Solution
|