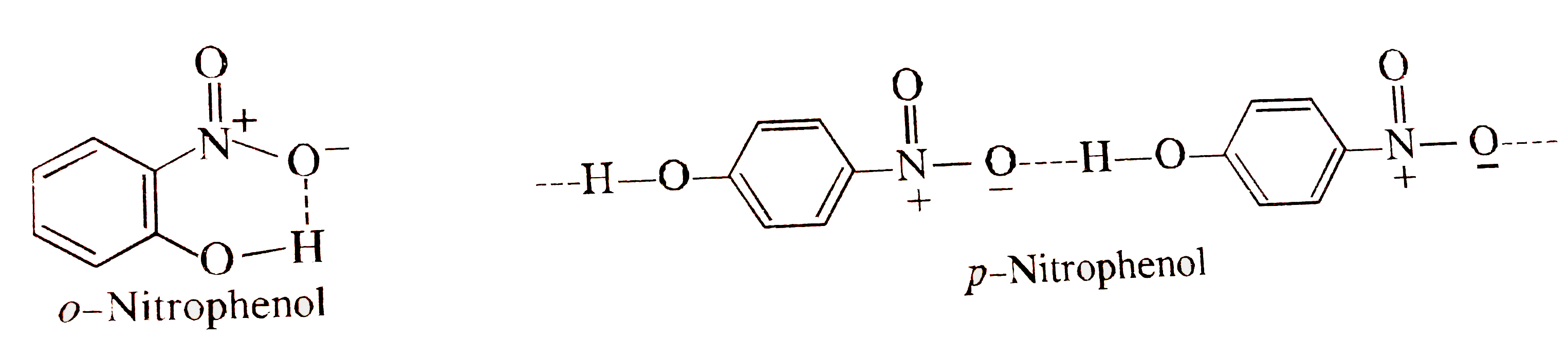Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise ADDITIONAL IMPORTANT QUASTIONS|41 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise H.O.T.S. CONCEPTUAL QUESTIONS|11 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise COMPREHENSION|4 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
DINESH PUBLICATION|Exercise Unit test|20 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
DINESH PUBLICATION|Exercise All Questions|310 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -BORD EXAMINATIONS
- What is the type of hybridisation of carbon atoms marked with star. ...
Text Solution
|
- A molecule of H(2) exist while that of the He(2) does not. Explain.
Text Solution
|
- NaCl and AgNO(3) are ionic solids and they readily dissodciate to from...
Text Solution
|
- BeF(2) and H(2)O are both tri-atomic molecules but have different shap...
Text Solution
|
- Bond angle in NH(3) is molre than in PH(3). Explain.
Text Solution
|
- Why is HCl predominantly covalent in the gaseous state but is ionic in...
Text Solution
|
- Can a non-polar molecule have polar covalent bonds?
Text Solution
|
- (a) Explain sigma and pi bonds with suitable examples, (b) A pi bond...
Text Solution
|
- PCl(5) exists but NCl(5) does not because
Text Solution
|
- o- nitrophenol is steam volatile while p-nitrophenol is not. Discuss.
Text Solution
|
- KHF(2) exists while KHCl(2) does not. Explain.
Text Solution
|
- Yor are given the electronic configuration of five neural atoms -A,B,C...
Text Solution
|
- Boiling point of ethane is more than that of methane. Assign reason.
Text Solution
|
- Which comopound from each of the following pairs ismore covalent and w...
Text Solution
|
- The bond angles in NH(4)^(+) and CH(4) are same but NH(3) has differen...
Text Solution
|
- Predict which out of the following species are planar. (i) NH(4)^(+)...
Text Solution
|
- Why is MgCl(2) molecule linear whereas the molecules of SnCl(2) chlori...
Text Solution
|
- The hybridisatio of oxygen in both water and diethyl ether molecules i...
Text Solution
|
- Both Na and H occur in group 1 of the periodic table ,yet melting poin...
Text Solution
|
- With the help of molecular orbital theory, draw the molecular orbital ...
Text Solution
|