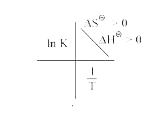
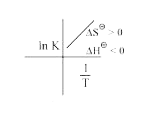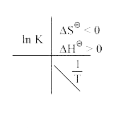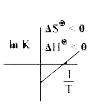A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the plots of ln K vs (1/T) is/are correct?
Text Solution
|
- Draw the plot log V vs log T.
Text Solution
|
- The plot og log k vs 1//T helps to calculate
Text Solution
|
- A graph plotted between log k vs (1)/(T) is represented by
Text Solution
|
- Select the incorrect statements about the plots of ln K vs 1/T
Text Solution
|
- Which of the plots of ln K vs (1/T) is/are correct?
Text Solution
|
- The plot of log k vs 1/T helps to calculate
Text Solution
|
- The plot of log k vs 1/T helps to calculate
Text Solution
|
- Which is the correct plot for In k vs 1/T?
Text Solution
|