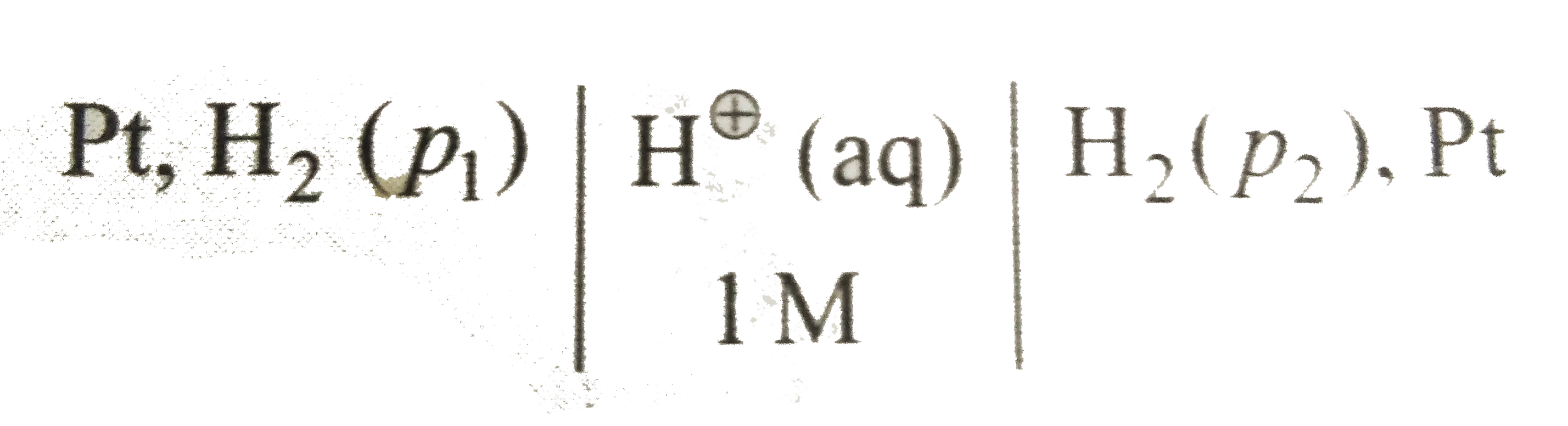A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-ELECTROCHEMISTRY-EXERCISE-4,I
- Consider the following cell with hydrogen electrodes at difference pre...
Text Solution
|
- Which of the following reaction is possible at anode ?
Text Solution
|
- Refining of impure copper with zinc impurity is to be done by electrol...
Text Solution
|
- For a cell given below Ag|Ag^(+)||Cu^(2+)|Cu Ag^(+)+e^(-)toAg,E^(@...
Text Solution
|
- For a cell reaction involving a two-electron change, the standard e.m...
Text Solution
|
- Stadard reduction electrode potentials of three metals A,B and C are r...
Text Solution
|
- For the redox reaction: Zn(s) +Cu^(2+) (0.1M) rarr Zn^(2+) (1M)+Cu(s...
Text Solution
|
- When during electrolusis of a solution of AgNO3, 9650 coulmbs of char...
Text Solution
|
- Consider the following E^o values : E^o Fe^(3+)//FE^(2+)o = + 0.77 ...
Text Solution
|
- The standard e.m.f. of a cell, invoving one electron change is found t...
Text Solution
|
- The limiting molar conductivities Lambda^@ for NaCL, KBr and KCI are ...
Text Solution
|
- In a cell that untillizes the reaction , Zn((s)) + 2 H((aq))^+ rarr ...
Text Solution
|
- Aluminium oxide may be electrolysed at 1000^(@)C to furnish aluminium ...
Text Solution
|
- For a spontaneous reaction the DeltaG, equilibrium constant (K) and E(...
Text Solution
|
- Given the data at 25^(@)C Ag+I^(-)rarrAgl+e^(-)" "E^(@)=0.153V A...
Text Solution
|
- The cell , Zn | Zn^(2+) (1M) || Cu^(2+) (1M) Cu (E("cell")^@ = 1. 10 V...
Text Solution
|
- The equivalent conductances of two strong electrolytes at infinite di...
Text Solution
|
- Given E(Cr^(3+)//Cr)^(@)=-0.72V, and E(Fe^(2+)//Fe)^(@)=-0.42V The...
Text Solution
|
- The standard reduction potential for Zn^(2+)//Zn, Ni^(2+)//Ni and Fe^(...
Text Solution
|
- Given E(cr^(3+)//Cr)^(0)=-0.74 V, E(MnO(4)//Mn^(2+))^(0)=1.51 cm E...
Text Solution
|