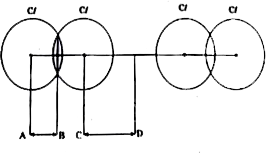
Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VGS PUBLICATION-BRILLIANT|Exercise Short Answer Questions|41 VideosCHEMICAL EQUILIBRIUM AND ACIDS-BASES
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTIONS & ANSWERS|8 VideosENVIRONMENTAL CHEMISTRY
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|6 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -Long Answer Questions
- Write an essay on s, p, d and f-block elements.
Text Solution
|
- Relate the electronic configuration of elements and their properties i...
Text Solution
|
- What is a periodic property? How the following properties vary in a gr...
Text Solution
|
- What is a periodic property? How the following properties vary in a gr...
Text Solution
|
- What is a periodic property? How the following properties vary in a gr...
Text Solution
|
- What is a periodic property? How the following properties vary in a gr...
Text Solution
|
- Write a note on (a) Atomic radius.
Text Solution
|
- Write a note on (b) Metallic radius.
Text Solution
|
- Write a note on (c ) Covalent radius.
Text Solution
|
- Define IE(1) and IE(2). Why is IE(2) > IE(1) for a given atom? Discuss...
Text Solution
|
- How do the following properties change in group - 1 and in the third p...
Text Solution
|
- How do the following properties change in group - 1 and in the third p...
Text Solution
|
- How do the following properties change in group - 1 and in the third p...
Text Solution
|
- How do the following properties change in group - 1 and in the third p...
Text Solution
|
- Define electron gain enthalpy. How it varies in a group and in a perio...
Text Solution
|
- What is electronegativity?
Text Solution
|
- How does it very in a group and in a period ?
Text Solution
|
- Explain the following (a) Valency
Text Solution
|
- Explain the following (b) Diagonal relationship
Text Solution
|
- Explain the following (c ) Variation of nature of oxides in the Gro...
Text Solution
|