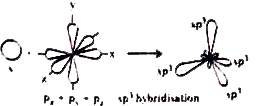Hybridisation : "intermixing of atomic orbitals of almost equal energies of an atom and their redistribution into an equal number of identical orbitals is called, 'hybridisation'.
Examples : Let us see the three types of hybridisations - `sp^(3), sp^(2)` and sp.
1) `sp^(3)` Hybridisation : The phenomenon of intermixing of one 's' orbital and three 'p' orbitals forming four `'sp^(3)'` hybrid orbitals is called `sp^(3)` hybirdisation.
Each of the `sp^(3)` hybrid orbitals possess `1/4` of s - character and `3/4` of p character. The bond angle in-betweeen any two `sp^(3)` hybrid orbitals is `109^(@)28'`. The shape of the molecule in which the central atom undergoes `sp^(3)` hybridisaition is tetrahedral.
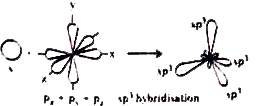
Example : Formation of Methane `(CH_(4))` molecule :
In the formation of methane molecule the central carbon atom undergoes `sp^(3)` hybridisation in its excited state. As a result of which four `sp^(3)` hybrid orbitals will form on it. All the four `sp^(3)` hybrid orbitals possess bond pair of electrons. Now, the four `sp^(3)` hybrid orbitals overlap head - head with '1s' orbitals of four 'H' atoms forming `CH_(4)` molecule. The shape of the molecule is tetrahedral with a bond angle of `109^(@)28'`.
C(Z) = 6 `" " 1s^(2)2s^(2)2p^(2)`
Ground state `" " 1s^(2)2s^(2)2p^(1)2p^(1)`
Excited state `" "underbrace(1s^(2)2s^(1)2p_(x)^(1)2p_(y)^(1)2p_(z)^(1))_(sp^(3)`

2) `sp^(2)` Hybridisation : The phenomenon of intermixing of one 's' orbital and two 'p' orbitals forming three `'sp^(2)'` hybrid orbitals is called `sp^(2)` hybridisation.
Each of the `sp^(2)` hybrid orbitals possesses `1/3` s - character and `2/3` p - character. The bond angle in between any two `sp^(2)` hybrid orbitals is `120^(@)`. The shape of the molecule in which the central atom undergoes `sp^(2)` hybridisation is plane triangular.

Example : Formation of Boron trichloride `(BCl_(3))` molecule :
In the formation of `BCl_(3)` molecule the central boron atom undergoes `sp^(2)` hybridisation in its excited state `(1s^(2)2s^(1)2p_(x)^(1)2p_(y)^(1)2p_(z)^(0))`. As a result of which three `sp^(2)` hybrid orbitals will form on it. All the three hybrid orbitals possess an electron each. Now, the three `sp^(2)` hybrid orbitals overlap head - head with half - filled `'3p_(z)` orbitals of three chlorine atoms forming `BCl_(3)` molecule. The shape of the molecule is plane triangular with a bond angle of `120^(@)`.

3) sp Hybridisation : The phenomenon of intermixing of one 's' orbital and one 'p' orbital of an atom forming two 'sp' hybrid orbitals is called sp hybridisation.
Each of the sp hybrid orbitals possesses `1/2` s - character and `1/2` p - character. The bond angle in-between the two hybrid orbitals is `180^(@)`. The shape of the molecule in which the central atom undergoes sp hybridisation is linear.

Example : Formation of Beryllium chloride `(BeCl_(2))` :
In the formation of `BeCl_(2)` molecule the central 'Be' atom undergoes sp hybridisation in its excited state `(1s^(2)2s^(1)2p_(x)^(1)2p_(y)^(0)2p_(z)^(0))`. As a result of which two sp hybrid orbitals will form on it. The two hybrid orbitals contain an electron, each. Now, the two sp hybrid orbitals overlap head - head with half - filled `'3p_(z)'` orbitals of two chlorine atoms forming `BeCl_(2)` molecule. The shape of the molecule is linear with a bond angle is `180^(@)`.