Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS - GROUP 13
VGS PUBLICATION-BRILLIANT|Exercise SHORT ANSWER QUESTIONS|27 VideosTHE P-BLOCK ELEMENTS - GROUP 13
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|3 VideosSTOICHIOMETRY
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTIONS & ANSWERS|10 VideosTHE p-BLOCK ELEMENTS-GROUP 14
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|7 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-THE P-BLOCK ELEMENTS - GROUP 13 -LONG ANSWER QUESTIONS
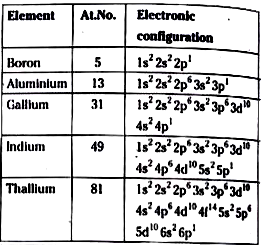
- Write the electronic configuration of group-13 elements .
Text Solution
|
- How are borax and boric acid prepared ? Explain the action of heat on ...
Text Solution
|
- How is diborane prepared ? Explain its structure .
Text Solution
|
- Write any two methods of preparation of diborane. How does it react wi...
Text Solution
|