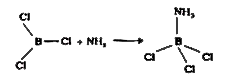Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS - GROUP 13
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|3 VideosTHE P-BLOCK ELEMENTS - GROUP 13
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|3 VideosSTOICHIOMETRY
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTIONS & ANSWERS|10 VideosTHE p-BLOCK ELEMENTS-GROUP 14
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|7 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-THE P-BLOCK ELEMENTS - GROUP 13 -SHORT ANSWER QUESTIONS
- Write reactions to justify amphoteric nature of aluminium .
Text Solution
|
- What are electron deficient compounds ? Is BCl3 an electron deficient ...
Text Solution
|
- Suggest reasons why the B-F bonds lengths in BF3 (130 pm) and BF4^- ...
Text Solution
|
- B-Cl bond has a bond moment. Explain why BCl3 molecule has zero dipole...
Text Solution
|
- Explain the structure of boric acid.
Text Solution
|
- What happens when Borax is heated strongly ?
Text Solution
|
- What happens when Boric acid is added to water ?
Text Solution
|
- What happens when Aluminium is heated with dilute NaOH ?
Text Solution
|
- What happens when BF3 is treated with ammonia ?
Text Solution
|
- What happens when Hydrated alumina is treated with aq.NaOH solution ?
Text Solution
|
- Give reasons Conc. HNO3 can be transported in aluminium container.
Text Solution
|
- Give reasons A mixture of dil. NaOH and aluminium pieces in used to...
Text Solution
|
- Give reasons Aluminium alloys are used to make aircraft body
Text Solution
|
- Give reasons Aluminium utensils should not be kept in water overnigh...
Text Solution
|
- Give reasons Aluminium wire is used to make transmission cables .
Text Solution
|
- Explain why the electronegativity of Ga, In and Tl will not vary very ...
Text Solution
|
- Explain borax bead test with a suitable example
Text Solution
|
- Explain the structure of diborane.
Text Solution
|
- Explain the reactions of aluminium with acids.
Text Solution
|
- Write a short note on the anomalous behaviour of boron in the group - ...
Text Solution
|