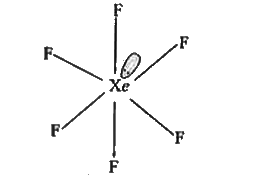
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SIA PUBLICATION-p-BLOCK ELEMENTS-MCQ.s
- The hybridisation of Xe and the number of lone pairs of electrons on i...
Text Solution
|
- Identify the most acidic oxide among the following oxides based on the...
Text Solution
|
- identify the correct statement
Text Solution
|
- Assertion (A): Noble gases have very low boiling points. Reason (R):...
Text Solution
|
- Which one of the following is correct with respect to basic character?
Text Solution
|
- Sea divers use a mixture of
Text Solution
|
- Which one of the following elements does not form triiodide on reactin...
Text Solution
|
- The buffer system which helps to maintain the pH of blood between 7.26...
Text Solution
|
- The key step in the manufacturing of H(2)SO(4) by contact process is
Text Solution
|
- Ammonia on reaction with chlorine forms an explosive NCl(3). What is t...
Text Solution
|
- The structure of XeOF(4) is
Text Solution
|
- Which one of the following correctly represents the variation of elect...
Text Solution
|
- Which one of the following elements reacts with steam?
Text Solution
|
- Which one of the following elements on doping with germanium, make it ...
Text Solution
|
- Diborane reacts with HCl in the present of AlCl(3) and liberates
Text Solution
|
- Which one of the following is not correct?
Text Solution
|
- Na(2)S(2)O(3) reacts with moist Cl(2) to form Na(2)SO(4), HCl and X. w...
Text Solution
|
- The role of copper diaphragm in Whytlaw-Gray's method is
Text Solution
|
- Liquid X is used is bubble chamber to detect neutral mesons and gamma ...
Text Solution
|
- Which of the following cannot form an amphoteric oxide?
Text Solution
|
- The catalyst and promoter respectively used in the Haber's process of ...
Text Solution
|