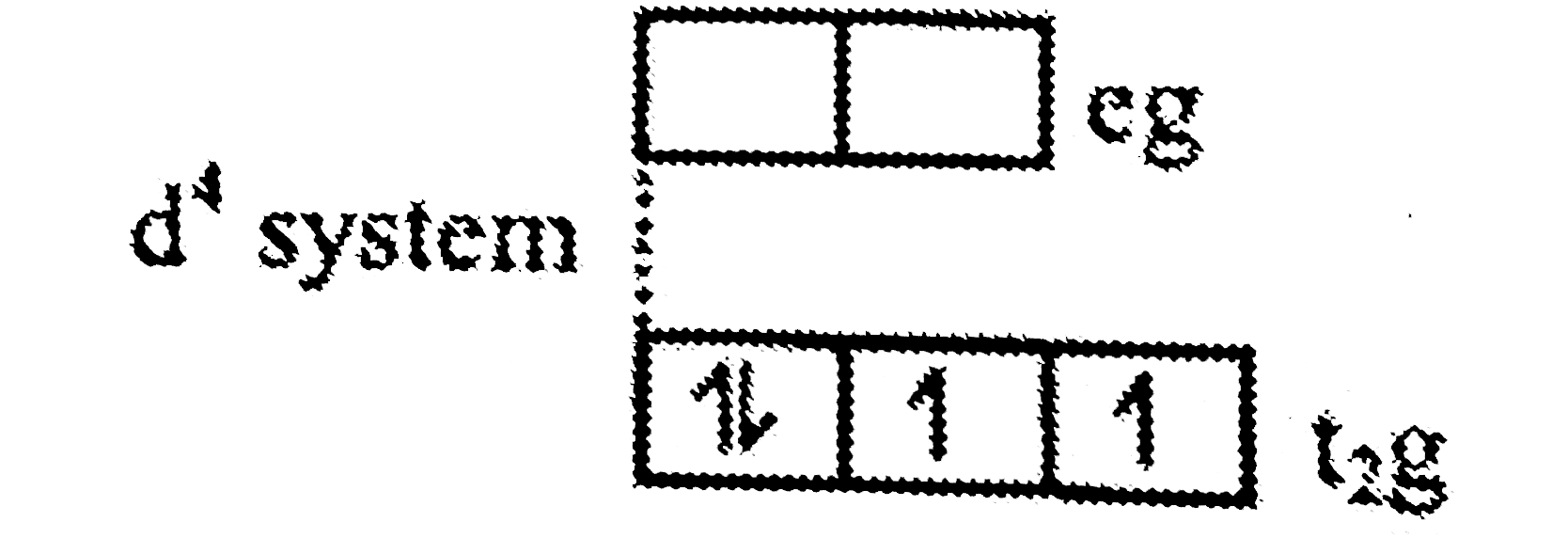
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CAREER POINT-MOCK TEST 10-PART (B) - CHEMISTRY
- Which of the following can react with both HCl and NaOH ?
Text Solution
|
- Which of the following solid has maximum melting points?
Text Solution
|
- The catalyst used in the manufacture of polyethylene Zeigler method is...
Text Solution
|
- The product C is -
Text Solution
|
- Iodine is powerful antiseptic. It is used as a tincture of iodine whic...
Text Solution
|
- Two elemets X( atomic weight =75) and Y( atomic weight =16) combine to...
Text Solution
|
- The value of the spin only magnetic moment for one of the following co...
Text Solution
|
- An element X (At,wt = 80 g//mol) having fcc structure, calculate the n...
Text Solution
|
- Which of the following donot give Cannizzaro reaction ?
Text Solution
|
- Consider the following reactions, The end product 'S' is -
Text Solution
|
- The IUPAC name of CH(3)-underset(COOC(2)H(5))underset(|)(C)=CH-CH(2)-o...
Text Solution
|
- The order of leaving group ability is .^(-)underset((I))(OAc).^(-) u...
Text Solution
|
- Often in water bodies subjected to sewage pollution, fishes die becaus...
Text Solution
|
- A solution containing 500 g of a protein per liter is isotonic with a...
Text Solution
|
- Calculate partial pressure of B at equilibrium in the following equili...
Text Solution
|
- What is the melting point of benzene if DeltaH("fusion")= 9.95kJ//mol ...
Text Solution
|
- D(2)O(Heavy water)" and "H(2)O differ in following except -
Text Solution
|
- Carborundum is -
Text Solution
|
- If degree of dissociation of 2M CH(3)COOH is 10% then degree of disso...
Text Solution
|
- The solubility in terms of K(sp) "for " A3B((aq)) is
Text Solution
|