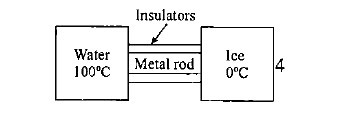
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HEAT TRANSFER
PHYSICS GALAXY - ASHISH ARORA|Exercise Unsolved Numerical Problems for Preparation of NSEP,INPhO&IPhO|66 VideosHEAT TRANSFER
PHYSICS GALAXY - ASHISH ARORA|Exercise Numerical MCQs Single Options Correct|94 VideosHEAT AND THERMAL EXPANSION
PHYSICS GALAXY - ASHISH ARORA|Exercise UNSOLVED NUMRICAL PROBLEMS FOR PREPARATION OF NSEP, INPhO & IPhO|82 VideosKinetic Theory of Gases and Gas Laws
PHYSICS GALAXY - ASHISH ARORA|Exercise Unsolved Numerical Problems for Preparation of NSEP, INPhO & IPhO|64 Videos
Similar Questions
Explore conceptually related problems
PHYSICS GALAXY - ASHISH ARORA-HEAT TRANSFER -Advance MCQs with One or More Option Correct
- An insulated container is filled with ice at 0^(@)C, and another conta...
Text Solution
|
- Two spheres A and B have radius but the heat capacity of A is greater ...
Text Solution
|
- The two ends of a uniform rod of thermal conductivity k are maintained...
Text Solution
|
- A planet having surface temperature T, K has a solar constant S. An an...
Text Solution
|
- Two ends of area A of a uniform rod of thermal conductivity k are main...
Text Solution
|
- Two bodies A and B have thermal emissivities of 0.01 and 0.81 respecti...
Text Solution
|
- Curved surface of a uniform rod is isolated from surrounding. Ends of ...
Text Solution
|
- A thin spherical shell and a thin cylindrical shell (closed at both en...
Text Solution
|
- The gross radiation emitted by a perfectly black body is:
Text Solution
|
- The rates of fall temperature of two identical solid spheres of differ...
Text Solution
|
- A hollow copper sphere & a hollow copper cube of same surface area & n...
Text Solution
|
- The plots of intensity vs wavelength for two black bodies at temperatu...
Text Solution
|
- A metal cylinder of mass 0.5 kg is heated electrically by a 12 W heate...
Text Solution
|
- A particle of mass 1 kg slides in a horizontal circle of radius 20 m w...
Text Solution
|
- In accordance with Kirchhoffs law (Assume transmissivity a(t)arrow 0 f...
Text Solution
|
- A hollow and a solid sphere of same material and identical outer surfa...
Text Solution
|
- A heated body emits radiation which has maximum intensity at frequency...
Text Solution
|
- Two spherical black bodies A and B,having radii r(A) and r(B) =2r(A) e...
Text Solution
|
- Which of the following statements are true?
Text Solution
|
- A 100 cm long cylindrical flask with inner and outer diameter 2 cm and...
Text Solution
|