A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 40-CHEMISTRY
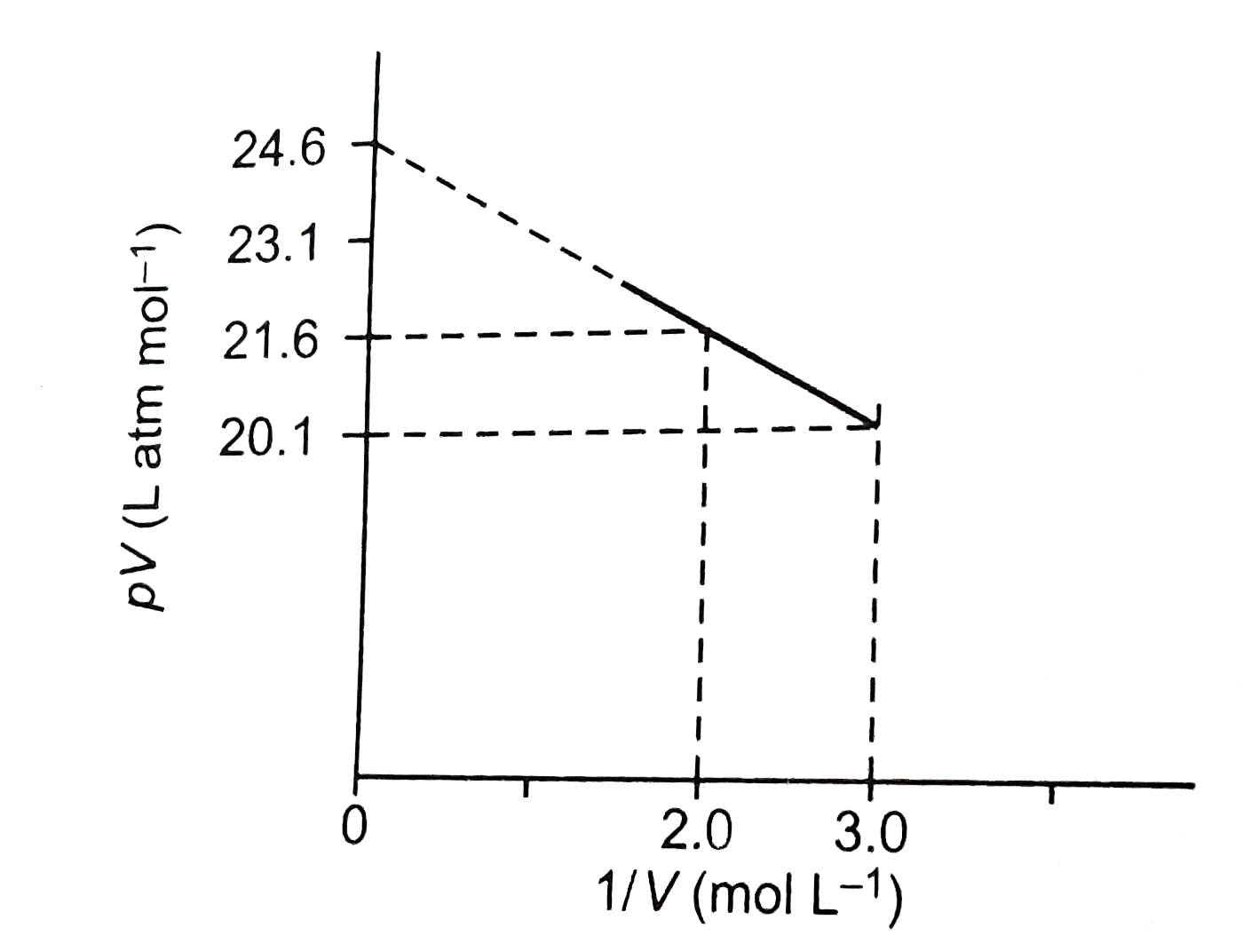
- For one mole of a van der Waals' gas when b=0 and T=300K, the pV vs 1/...
Text Solution
|
- The kinetic energy of an electron in the second Bohr orbit of a hydrog...
Text Solution
|
- With respect to graphite and diamond, which of the statements given be...
Text Solution
|
- Two moles of an ideal gas is expanded isothermally and reversibly from...
Text Solution
|
- AgNO(3)(aq.) was added to an aqeous KCl solution gradually and the con...
Text Solution
|
- The bond dissociation energy of B-F in BF(3) is "646 kJ mol"^(-1) wher...
Text Solution
|
- The number of optically active products obtained from the complete ozo...
Text Solution
|
- The number of aldol reaction(s) that occurs in the given transformatio...
Text Solution
|
- The hyperconjugative stabilities of tert-butyl cation and 2-butene, re...
Text Solution
|
- The major product in the follo with conversion is
Text Solution
|
- Nitration of aniline in strong acidic medium also gives m-nitroaniline...
Text Solution
|
- 1g of a non-volatile non-electrolyte solute is dissolved in 100g of tw...
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- Amylopectin in composed of:
Text Solution
|
- Extra pure N2 can be obtained by heating
Text Solution
|
- If the dipole moment of AB molecule is given by 1.2 D and A - B the bo...
Text Solution
|
- Reduction of metal centre in aqueous permanganate ion involves (1) ...
Text Solution
|
- The equilibrium Cu^(0)+Cu^(||)hArr2Cu^(|) In the aqueous medium at 2...
Text Solution
|
- The correct order of the spin - only magnetic moments of the following...
Text Solution
|
- The volume (in mL) of 0.1M Ag NO(3) required for complete precipitatio...
Text Solution
|